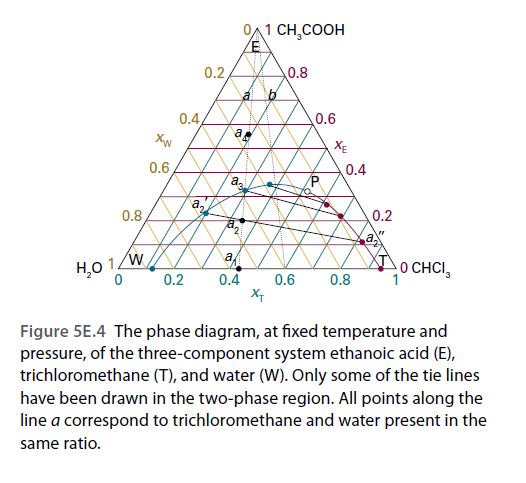
OA1 CH,COOH /E 0.2, 0.8 0.4 0.6 XE 0.6 0.4 a, 0.8 0.2 1ZW 0.2 a H,O 0 CHCI, 1 0.4 0.6 X- 0.8 Figure 5E.4 The phase diagram, at fixed temperature and pressure, of the three-component system ethanoic acid (E), trichloromethane (T), and water (W). Only some of the tie lines have been drawn in the two-phase region. All points along the line a correspond to trichloromethane and water present in the same ratio.
OA1 CH,COOH /E 0.2, 0.8 0.4 0.6 XE 0.6 0.4 a, 0.8 0.2 1ZW 0.2 a H,O 0 CHCI, 1 0.4 0.6 X- 0.8 Figure 5E.4 The phase diagram, at fixed temperature and pressure, of the three-component system ethanoic acid (E), trichloromethane (T), and water (W). Only some of the tie lines have been drawn in the two-phase region. All points along the line a correspond to trichloromethane and water present in the same ratio.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.3E
Related questions
Question
Refer to the ternary phase diagram in Fig. 5E.4. How many phases are present, and what are their compositions and relative abundances, in a mixture that contains 55.0 g of water, 8.8 g of trichloromethane, and 3.7 g of ethanoic acid? Describe what happens when (i) water, (ii) ethanoic acid is added to the mixture.
Transcribed Image Text:OA1 CH,COOH
/E
0.2,
0.8
0.4
0.6
XE
0.6
0.4
a,
0.8
0.2
1ZW
0.2
a
H,O
0 CHCI,
1
0.4
0.6
X-
0.8
Figure 5E.4 The phase diagram, at fixed temperature and
pressure, of the three-component system ethanoic acid (E),
trichloromethane (T), and water (W). Only some of the tie lines
have been drawn in the two-phase region. All points along the
line a correspond to trichloromethane and water present in the
same ratio.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 9 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
