Objectives: a) Define Aufbau principle, Pauli Exclusion Principle, and Hund's Rule. b) List and describe the four quantum numbers. c) Count valence electrons d) Write electron configurations 1-2) The Atomic Hotel I. The Atomic Hotel is a special hotel designed for electrons. The hotel has a strict policy called the Aufbau principle that states "ground" floors must be filled first and in order. It costs more to get rooms on higher floors. In the atomic world energy is money so "excited" electrons get rooms on higher floors, thus the exception to hotel policy. Looking back through the old guest logs the following layouts can be sketched. Determine which electrons had more "money". Label those electrons as excited. 1 1 1L 1L 1L May 23, 1998 June 19, 1999 September 10, 1991 A B
Objectives: a) Define Aufbau principle, Pauli Exclusion Principle, and Hund's Rule. b) List and describe the four quantum numbers. c) Count valence electrons d) Write electron configurations 1-2) The Atomic Hotel I. The Atomic Hotel is a special hotel designed for electrons. The hotel has a strict policy called the Aufbau principle that states "ground" floors must be filled first and in order. It costs more to get rooms on higher floors. In the atomic world energy is money so "excited" electrons get rooms on higher floors, thus the exception to hotel policy. Looking back through the old guest logs the following layouts can be sketched. Determine which electrons had more "money". Label those electrons as excited. 1 1 1L 1L 1L May 23, 1998 June 19, 1999 September 10, 1991 A B
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 30QAP: Consider the following representation of a set of p orbitals for an atom: mg...
Related questions
Question
Help pls. Tysm
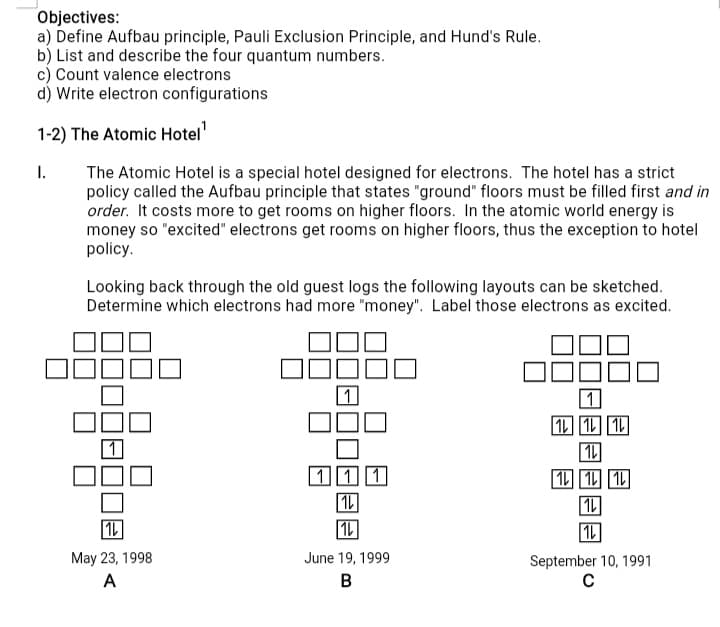
Transcribed Image Text:Objectives:
a) Define Aufbau principle, Pauli Exclusion Principle, and Hund's Rule.
b) List and describe the four quantum numbers.
c) Count valence electrons
d) Write electron configurations
1-2) The Atomic Hotel
The Atomic Hotel is a special hotel designed for electrons. The hotel has a strict
policy called the Aufbau principle that states "ground" floors must be filled first and in
order. It costs more to get rooms on higher floors. In the atomic world energy is
money so "excited" electrons get rooms on higher floors, thus the exception to hotel
policy.
I.
Looking back through the old guest logs the following layouts can be sketched.
Determine which electrons had more "money". Label those electrons as excited.
1
1
1L 1L 1L
May 23, 1998
June 19, 1999
September 10, 1991
A
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co