Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 90AP: Two possible Lewis diagrams for sulfine (H2CSO) are (a) Compute the formal charges on all atoms. (b)...
Related questions
Question
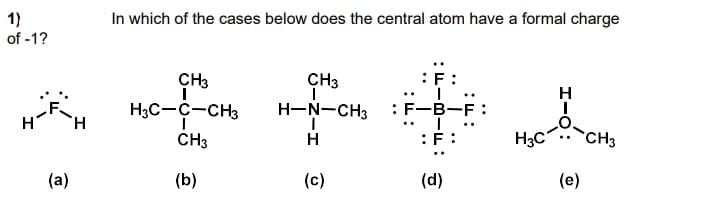
Transcribed Image Text:In which of the cases below does the central atom have a formal charge
1)
of -1?
CH3
CH3
:F:
H.
H3C-C-CH3
H-N-CH3
:F-B-F :
H.
CH3
:F:
H3C
CH3
H
(a)
(b)
(c)
(d)
(e)
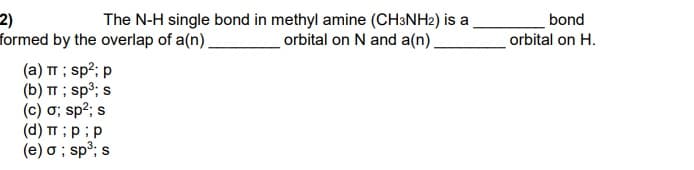
Transcribed Image Text:2)
formed by the overlap of a(n).
The N-H single bond in methyl amine (CH3NH2) is a
orbital on N and a(n),
bond
orbital on H.
(a) TT ; sp?; p
(b) TT ; sp³; s
(c) ơ; sp2; s
(d) TT ;p;p
(e) o ; sp³; s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning