of the assigned 1:1 buffer solution in a clean, labeled 100 mL Obtain 30 mL Make of the and stock solutions. Measure the of these solutions. Dilute the buffer solution in a 50.0 mL by a factor of by adding a little followed by of the buffer solution. Carefully fill to the line with and invert 10 times to mix and record the the solution. Pour the resulting solution into a clean solutions making sure to clean and rinse all glassware between uses. In your pre-lab, prepare a similar Repeat for the other two procedure for making the -fold dilution.
of the assigned 1:1 buffer solution in a clean, labeled 100 mL Obtain 30 mL Make of the and stock solutions. Measure the of these solutions. Dilute the buffer solution in a 50.0 mL by a factor of by adding a little followed by of the buffer solution. Carefully fill to the line with and invert 10 times to mix and record the the solution. Pour the resulting solution into a clean solutions making sure to clean and rinse all glassware between uses. In your pre-lab, prepare a similar Repeat for the other two procedure for making the -fold dilution.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter11: Solutions
Section: Chapter Questions
Problem 26P
Related questions
Question
please help with all parts of this question. DOUBLE CHECK YOUR ANSWERS AS PREVIOUS TUTORS GOT IT WRONG.
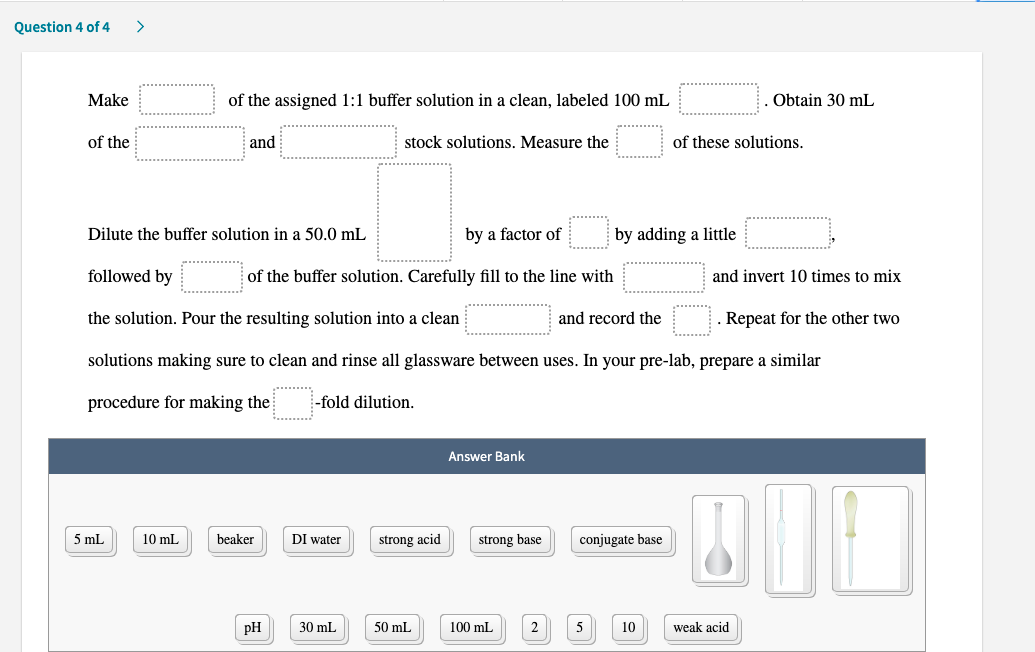
Transcribed Image Text:of the assigned 1:1 buffer solution in a clean, labeled 100 mL
Obtain 30 mL
Make
of the
and
stock solutions. Measure the
of these solutions.
Dilute the buffer solution in a 50.0 mL
by a factor of
by adding a little
followed by
of the buffer solution. Carefully fill to the line with
and invert 10 times to mix
and record the
the solution. Pour the resulting solution into a clean
solutions making sure to clean and rinse all glassware between uses. In your pre-lab, prepare a similar
Repeat for the other two
procedure for making the
-fold dilution.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co