OH is a very poor leaving group. However, many alcohols react with alkyl or aryl sulfo- nyl chlorides to give sulfonate esters. R-OH + R'- C - R,N >R-O-S-R' + HCI Suggest the product of the following reaction. CH,CH,-0-S-C,H; + CH,S¯N * DMSO
OH is a very poor leaving group. However, many alcohols react with alkyl or aryl sulfo- nyl chlorides to give sulfonate esters. R-OH + R'- C - R,N >R-O-S-R' + HCI Suggest the product of the following reaction. CH,CH,-0-S-C,H; + CH,S¯N * DMSO
Chapter16: Chemistry Of Benzene: Electrophilic Aromatic Substitution
Section16.SE: Something Extra
Problem 30MP: The carbocation electrophile in a Friede1-Crafts reaction can be generated by an alternate means...
Related questions
Question
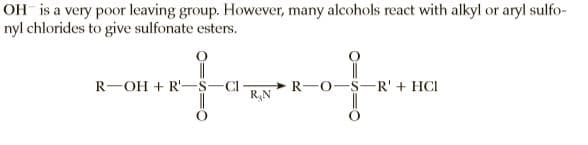
Transcribed Image Text:OH is a very poor leaving group. However, many alcohols react with alkyl or aryl sulfo-
nyl chlorides to give sulfonate esters.
R-OH + R'-
C -
R,N
>R-O-S-R' + HCI
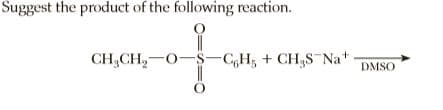
Transcribed Image Text:Suggest the product of the following reaction.
CH,CH,-0-S-C,H; + CH,S¯N *
DMSO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

