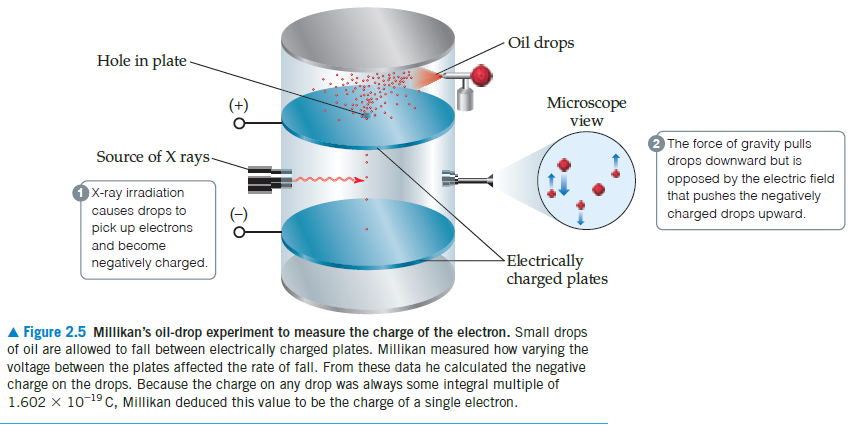
Oil drops Hole in plate Microscope view (+) The force of gravity pulls drops downward but is opposed by the electric field that pushes the negatively charged drops upward. Source of X rays- X-ray irradiation causes drops to pick up electrons (-) and become Electrically charged plates negatively charged. A Figure 2.5 Millikan's oil-drop experiment to measure the charge of the electron. Small drops of oil are allowed to fall between electrically charged plates. Millikan measured how varying the voltage between the plates affected the rate of fall. From these data he calculated the negative charge on the drops. Because the charge on any drop was always some integral multiple of 1.602 x 10-19 C, Millikan deduced this value to be the charge of a single electron.
Oil drops Hole in plate Microscope view (+) The force of gravity pulls drops downward but is opposed by the electric field that pushes the negatively charged drops upward. Source of X rays- X-ray irradiation causes drops to pick up electrons (-) and become Electrically charged plates negatively charged. A Figure 2.5 Millikan's oil-drop experiment to measure the charge of the electron. Small drops of oil are allowed to fall between electrically charged plates. Millikan measured how varying the voltage between the plates affected the rate of fall. From these data he calculated the negative charge on the drops. Because the charge on any drop was always some integral multiple of 1.602 x 10-19 C, Millikan deduced this value to be the charge of a single electron.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.84PAE: 2.84 Early attempts to arrange the elements often focused on atomic weight. Mendeleev considered a...
Related questions
Question
Go Figure Are the masses of the oil drops changed significantly when electrons
accumulate on them?
Transcribed Image Text:Oil drops
Hole in plate
Microscope
view
(+)
The force of gravity pulls
drops downward but is
opposed by the electric field
that pushes the negatively
charged drops upward.
Source of X rays-
X-ray irradiation
causes drops to
pick up electrons
(-)
and become
Electrically
charged plates
negatively charged.
A Figure 2.5 Millikan's oil-drop experiment to measure the charge of the electron. Small drops
of oil are allowed to fall between electrically charged plates. Millikan measured how varying the
voltage between the plates affected the rate of fall. From these data he calculated the negative
charge on the drops. Because the charge on any drop was always some integral multiple of
1.602 x 10-19 C, Millikan deduced this value to be the charge of a single electron.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax