Olt Cl Question 3. The boiling point of a molecule is determined by its formula weight and the types of functional groups it contains. Arrange the following compounds in order of decreasing boiling points. Explain your reasoning. a) CH3CH2CH3, CH3CH2CH2CH2CH2CH2CH2CH,CH2CH3, CH3CH2CH2CH2CH3 b) CH3CH2CH½CH3 or ОН 14 ets c) ОН or ОН ОН d) or он
Olt Cl Question 3. The boiling point of a molecule is determined by its formula weight and the types of functional groups it contains. Arrange the following compounds in order of decreasing boiling points. Explain your reasoning. a) CH3CH2CH3, CH3CH2CH2CH2CH2CH2CH2CH,CH2CH3, CH3CH2CH2CH2CH3 b) CH3CH2CH½CH3 or ОН 14 ets c) ОН or ОН ОН d) or он
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter12: Chirality
Section: Chapter Questions
Problem 34CTQ
Related questions
Question

Transcribed Image Text:Olt
Cl
Question 3.
The boiling point of a molecule is determined by its formula weight and the types of
functional groups it contains. Arrange the following compounds in order of decreasing
boiling points. Explain your reasoning.
a) CH3CH2CH3, CH3CH2CH2CH2CH2CH2CH2CH,CH2CH3, CH3CH2CH2CH2CH3
b) CH3CH2CH½CH3 or
ОН
14
ets
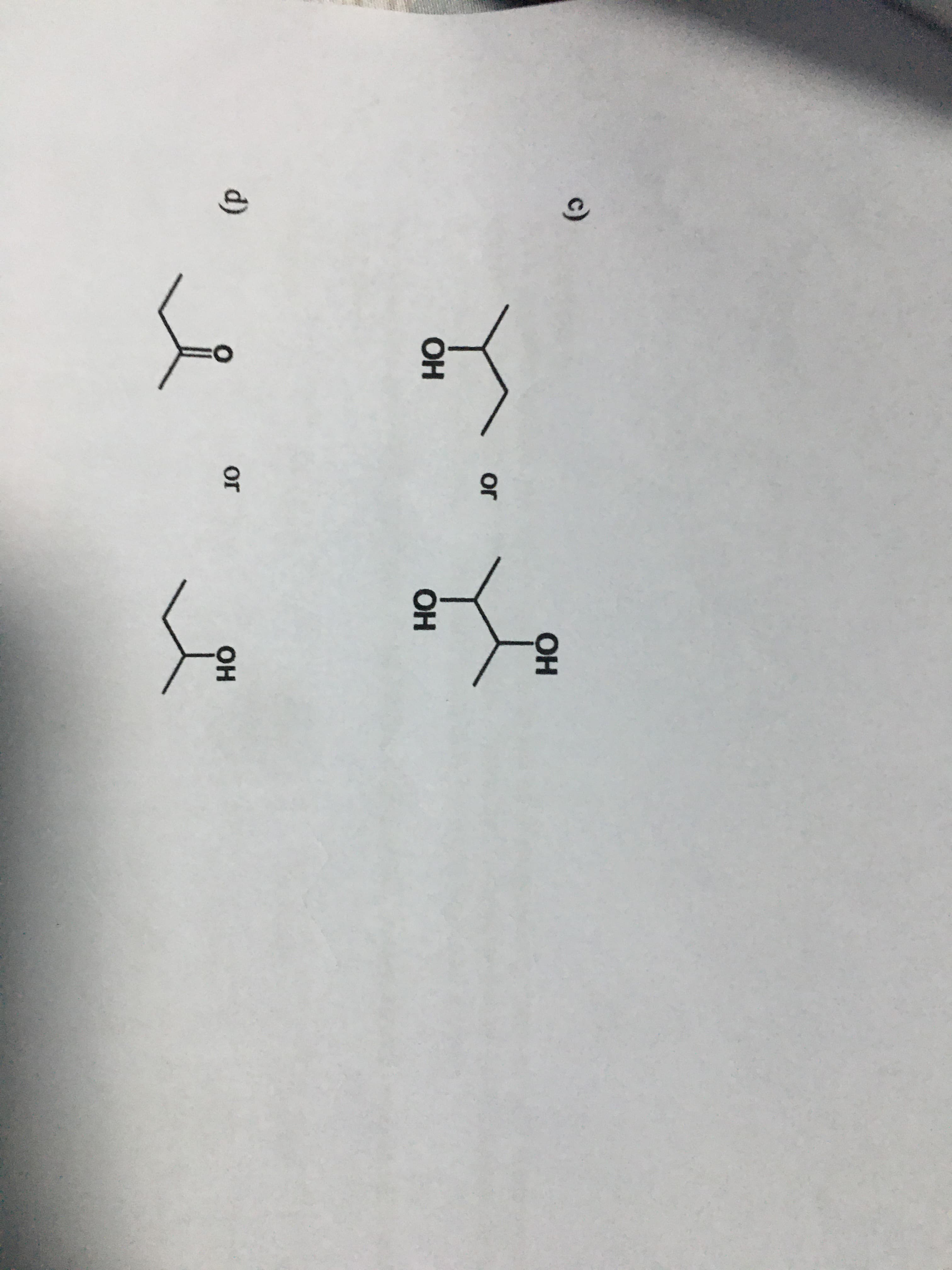
Transcribed Image Text:c)
ОН
or
ОН
ОН
d)
or
он
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning