In which of the following pure substances would hydrogen bonding be expected? Choose all that apply. H N-ethylpropanamide H- -H H propanal H- H ++ dimethyl ether H ethylamine H- H- H.
In which of the following pure substances would hydrogen bonding be expected? Choose all that apply. H N-ethylpropanamide H- -H H propanal H- H ++ dimethyl ether H ethylamine H- H- H.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter23: Organic Polymers, Natural And Synthetic
Section: Chapter Questions
Problem 10QAP
Related questions
Question
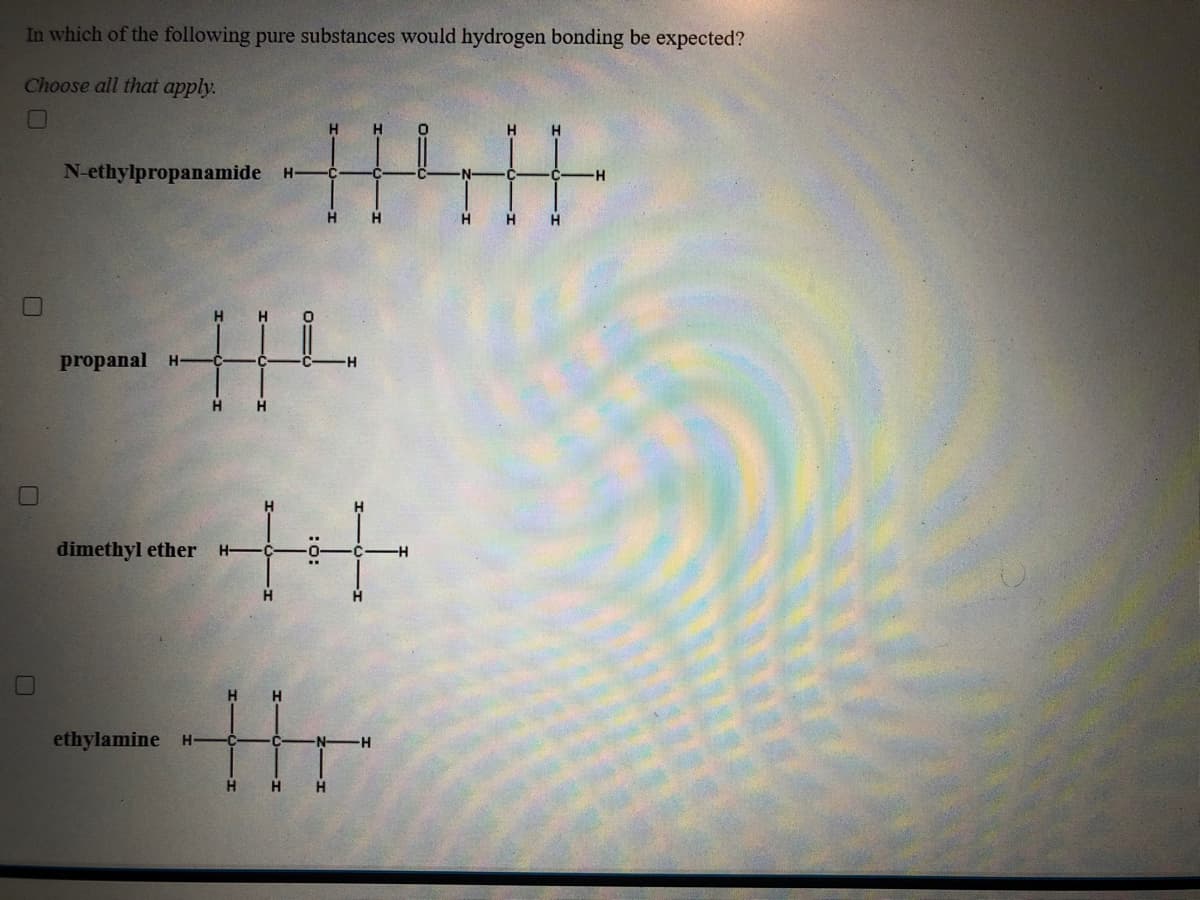
Transcribed Image Text:In which of the following pure substances would hydrogen bonding be expected?
Choose all that apply.
H
N-ethylpropanamide H-
-H
H
propanal
H
++
dimethyl ether
H
H.
ethylamine H-
H-
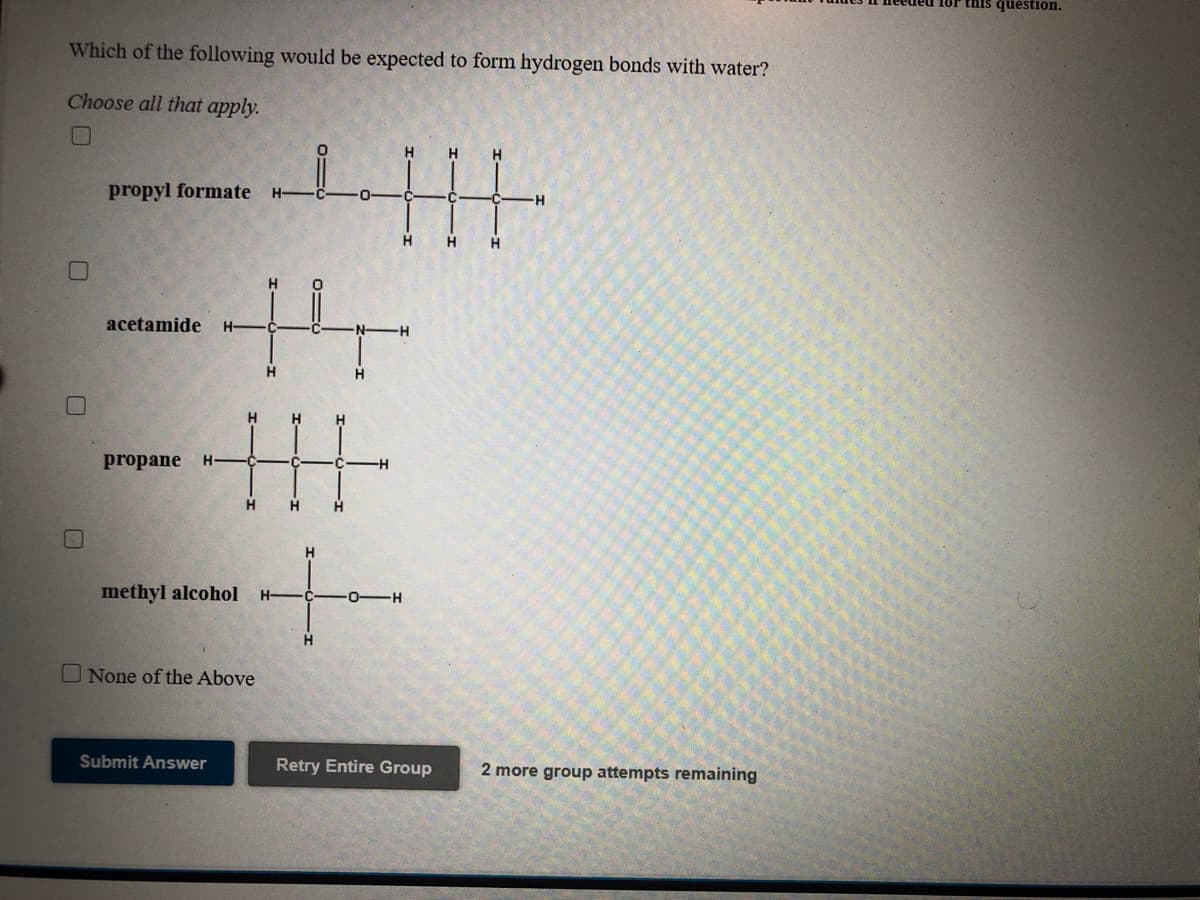
Transcribed Image Text:this question.
Which of the following would be expected to form hydrogen bonds with water?
Choose all that apply.
H
H
propyl formate
H-
-H
H
H.
acetamide
H-
H.
H
H
H.
pr
ne
H-
H.
H.
H
methyl alcohol
H-
O-
-H-
H
O None of the Above
Submit Answer
Retry Entire Group
2 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning