Omeprazole magnesium [(C17H18N3O3S)2 * Mg; MW = 713.12) dissociates to 70% at a certain concentration in solution. Calculate its dissociation factor. (Answer must be numeric. Do not include units. Round to one decimal place.) If 200 mg are contained within 100 g of an aqueous solution, what is the freezing point of the solution? (Answer must be numeric. Do not include units. If you answer is negative, then you must include the appropriate sign. Round to four decimal places.) °C Is the solution hypotonic, isotonic or hypertonic? (Answer must be typed)
Omeprazole magnesium [(C17H18N3O3S)2 * Mg; MW = 713.12) dissociates to 70% at a certain concentration in solution. Calculate its dissociation factor. (Answer must be numeric. Do not include units. Round to one decimal place.) If 200 mg are contained within 100 g of an aqueous solution, what is the freezing point of the solution? (Answer must be numeric. Do not include units. If you answer is negative, then you must include the appropriate sign. Round to four decimal places.) °C Is the solution hypotonic, isotonic or hypertonic? (Answer must be typed)
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 129CP: You make 20.0 g of a sucrose (C12H22O11) and NaCl mixture and dissolve it in 1.00 kg water. The...
Related questions
Question
Don't copy paste ans please
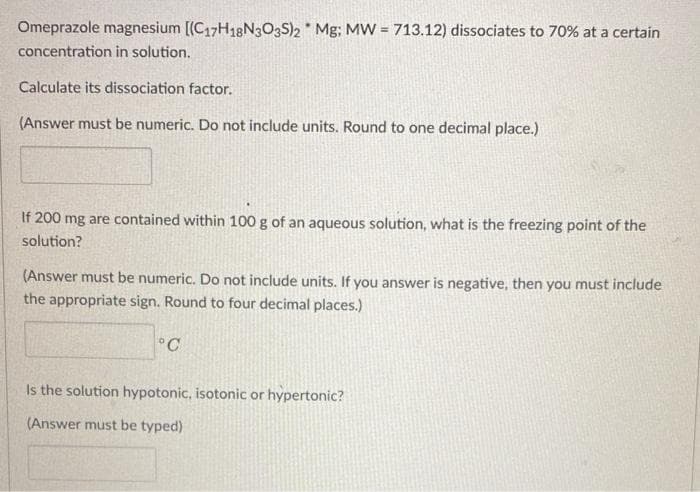
Transcribed Image Text:Omeprazole magnesium [(C17H18N3O3S)2 * Mg; MW = 713.12) dissociates to 70% at a certain
concentration in solution.
Calculate its dissociation factor.
(Answer must be numeric. Do not include units. Round to one decimal place.)
If 200 mg are contained within 100 g of an aqueous solution, what is the freezing point of the
solution?
(Answer must be numeric. Do not include units. If you answer is negative, then you must include
the appropriate sign. Round to four decimal places.)
°C
Is the solution hypotonic, isotonic or hypertonic?
(Answer must be typed)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning