World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter2: Matter
Section: Chapter Questions
Problem 7STP
Related questions
Question
Predict the product
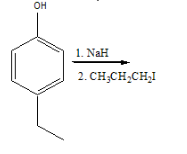
Transcribed Image Text:он
1. NaH
2. снсH-сHI
Expert Solution
Step 1
The given reaction is a Williamson ether synthesis.
Williamson ether synthesis is an organic reaction used to convert an alcohol and an alkyl halide to an ether using a base condition. This mechanism beings with the base, abstracting the proton from the alcohol to form an alkaoxide intermediate. This further reacts with an alkyl halide forming ether.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning