One type of breathalyzer employs a fuel cell to measure the quantity of alcohol in the breath. When a suspect blows into the breathalyzer, ethyl alcohol is oxidized to acetic acid at the anode: Part A CH3 CH2 OH(g) + 4 OH (aq) → HC, H3 O2 (9) + 3 H2O(1) + 4e At the cathode, oxygen is reduced: O2 (g) + 2H2O(1) + 4e 4 OH (aq) Assuming a pressure of 1.0 atm and a temperature of 25 °C, what percent (by volum driver's breath is ethanol? The overall reaction is the oxidation of ethyl alcohol to acetic acid and water. When a suspected drunk driver blows 191 mL of his breath through this breathalyzer, the breathalyzer produces an average of 329 mA of current for Express your answer using two significant figures. 10 s. % Submit Request Answer Provide Feedback
One type of breathalyzer employs a fuel cell to measure the quantity of alcohol in the breath. When a suspect blows into the breathalyzer, ethyl alcohol is oxidized to acetic acid at the anode: Part A CH3 CH2 OH(g) + 4 OH (aq) → HC, H3 O2 (9) + 3 H2O(1) + 4e At the cathode, oxygen is reduced: O2 (g) + 2H2O(1) + 4e 4 OH (aq) Assuming a pressure of 1.0 atm and a temperature of 25 °C, what percent (by volum driver's breath is ethanol? The overall reaction is the oxidation of ethyl alcohol to acetic acid and water. When a suspected drunk driver blows 191 mL of his breath through this breathalyzer, the breathalyzer produces an average of 329 mA of current for Express your answer using two significant figures. 10 s. % Submit Request Answer Provide Feedback
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter5: Electron Configurations And The Periodic Table
Section: Chapter Questions
Problem 3QRT
Related questions
Question
Transcribed Image Text:15:37 Sun May 2
Done <
AA
A session.masteringchemistry.com
<Problem Set #9 (Ch 19)
Exercise 19.114
14 of 15
I Review I Constants I Periodic Table
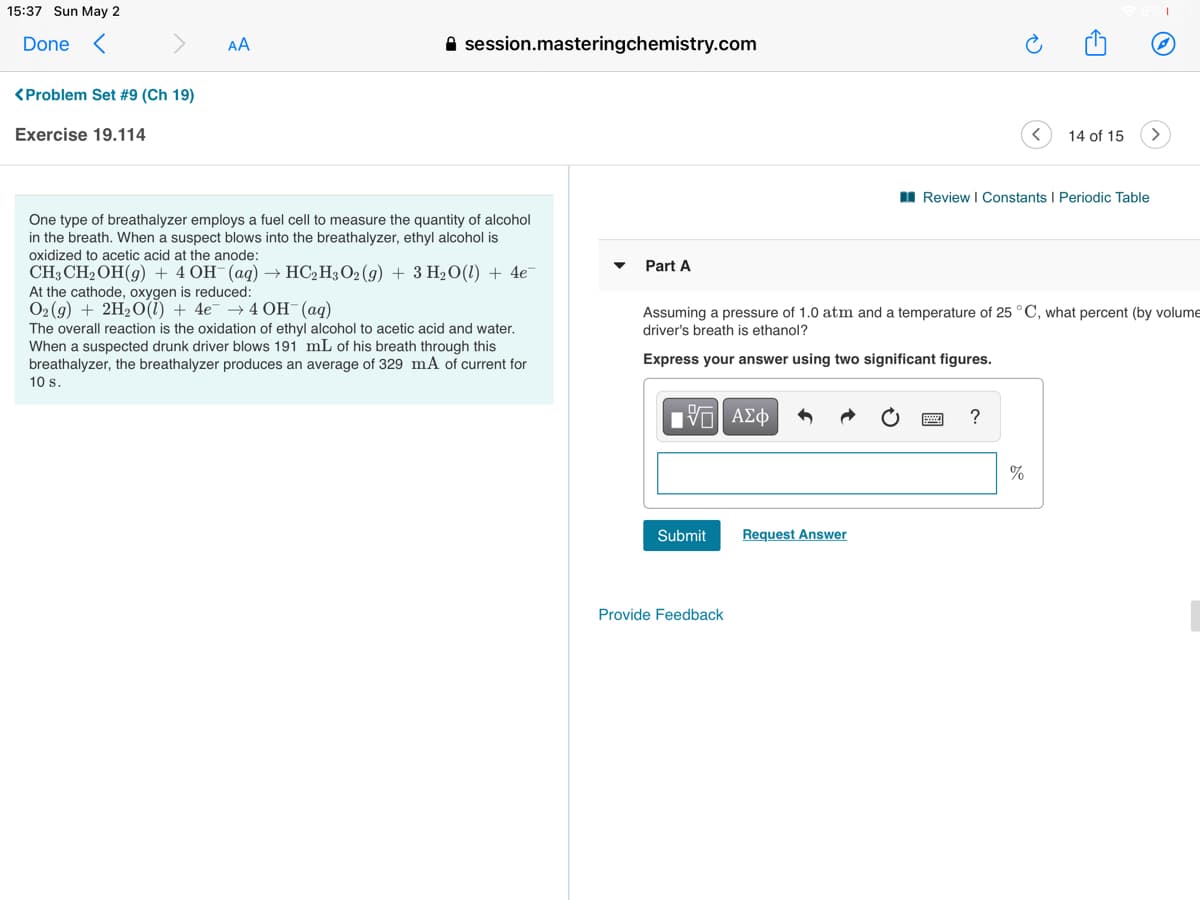
One type of breathalyzer employs a fuel cell to measure the quantity of alcohol
in the breath. When a suspect blows into the breathalyzer, ethyl alcohol is
oxidized to acetic acid at the anode:
Part A
CH3 CH2OH(g) + 4 OH-(aq) → HC,H3 O2 (g) + 3 H2O(1) + 4e
At the cathode, oxygen is reduced:
O2 (g) + 2H2O(1) + 4e¯ → 4 OH¯(aq)
Assuming a pressure of 1.0 atm and a temperature of 25 °C, what percent (by volume
driver's breath is ethanol?
The overall reaction is the oxidation of ethyl alcohol to acetic acid and water.
When a suspected drunk driver blows 191 mL of his breath through this
breathalyzer, the breathalyzer produces an average of 329 mA of current for
Express your answer using two significant figures.
10 s.
| ν ΑΣφ
?
Submit
Request Answer
Provide Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning