ons bartleby Fort Lewis College - CHEM 150 X + X p?id = 8911716 HW 6: Molecular Gec tes HW 6: Molecular Geometries Resources Hint If the symbolX represents a central atom, Y represents outer atoms, and Z represents lone pairs the structure Y-X-Y could be abbreviated as XY,Z,. on the central atom, Classify each molecule according to its shape. Linear Bent ( 120) Bent ( 109) Trigonal T-shaped See-saw Square planar Square pyramidal al ругar Answer Bank XY,ZZ XY,Z2 XY,Z XY,Z3 XY Z2 XY,Z XY,Z XY,Z about us privacy policy careers terms of use contact us help nno et al, 2 .pdf
ons bartleby Fort Lewis College - CHEM 150 X + X p?id = 8911716 HW 6: Molecular Gec tes HW 6: Molecular Geometries Resources Hint If the symbolX represents a central atom, Y represents outer atoms, and Z represents lone pairs the structure Y-X-Y could be abbreviated as XY,Z,. on the central atom, Classify each molecule according to its shape. Linear Bent ( 120) Bent ( 109) Trigonal T-shaped See-saw Square planar Square pyramidal al ругar Answer Bank XY,ZZ XY,Z2 XY,Z XY,Z3 XY Z2 XY,Z XY,Z XY,Z about us privacy policy careers terms of use contact us help nno et al, 2 .pdf
Chapter9: Covalent Bonding: Orbitals
Section: Chapter Questions
Problem 57E: Use Figs. 4-54 and 4-55 to answer the following questions. a. Would the bonding molecular orbital in...
Related questions
Question
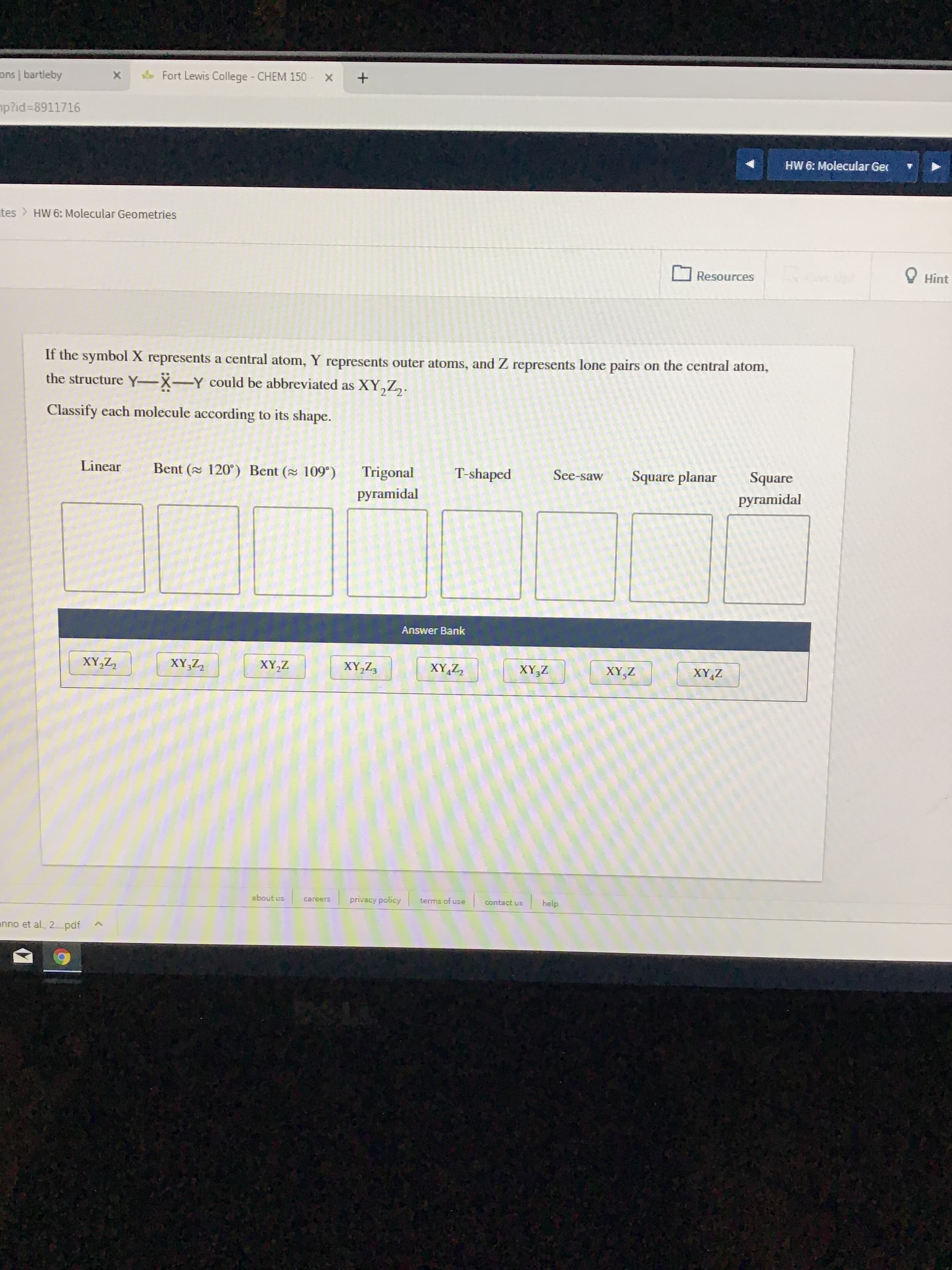
Transcribed Image Text:ons bartleby
Fort Lewis College - CHEM 150
X
+
X
p?id = 8911716
HW 6: Molecular Gec
tes
HW 6: Molecular Geometries
Resources
Hint
If the symbolX represents a central atom, Y represents outer atoms, and Z represents lone pairs
the structure Y-X-Y could be abbreviated as XY,Z,.
on the central atom,
Classify each molecule according to its shape.
Linear
Bent ( 120) Bent ( 109)
Trigonal
T-shaped
See-saw
Square planar
Square
pyramidal
al
ругar
Answer Bank
XY,ZZ
XY,Z2
XY,Z
XY,Z3
XY Z2
XY,Z
XY,Z
XY,Z
about us
privacy policy
careers
terms of use
contact us
help
nno et al, 2 .pdf
Expert Solution
Trending now
This is a popular solution!
Step by step
Solved in 9 steps with 9 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning