Open-Ended Questions A 20 dm' closed container containing 1.5 mol H, gas, x mol N, gas and 1.2 mol CO, gas has a total pressure of 500.0 kPa at a temperature of 298 K. (a) Calculate the value of x. (b) 1.5 mol of neon gas was added into the container. (1) (11) Calculate the total pressure of the resultant gas mixture. Calculate the partial pressures of all the component gases in the mixture.
Open-Ended Questions A 20 dm' closed container containing 1.5 mol H, gas, x mol N, gas and 1.2 mol CO, gas has a total pressure of 500.0 kPa at a temperature of 298 K. (a) Calculate the value of x. (b) 1.5 mol of neon gas was added into the container. (1) (11) Calculate the total pressure of the resultant gas mixture. Calculate the partial pressures of all the component gases in the mixture.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter8: Gases
Section: Chapter Questions
Problem 32Q
Related questions
Question
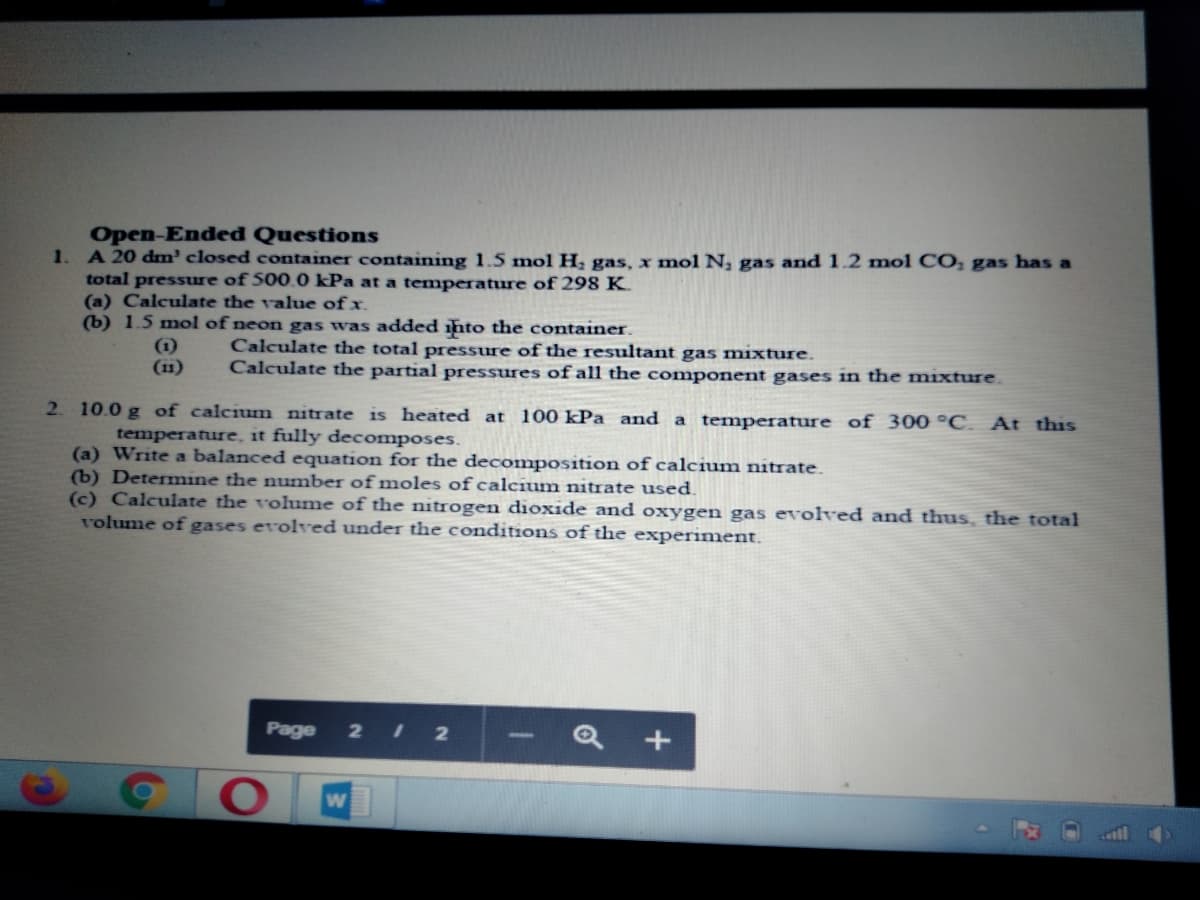
Transcribed Image Text:Open-Ended Questions
A 20 dm' closed container containing 1.5 mol H, gas,x mol N, gas and 1.2 mol CO, gas has a
total pressure of 500.0 kPa at a temperature of 298 K.
(a) Calculate the value of x.
(b) 1.5 mol of neon gas was added ihto the container.
1.
(1)
(11)
Calculate the total pressure of the resultant gas mixture.
Calculate the partial pressures of all the component gases in the mixture.
2 10.0 g of calcium nitrate is heated at 100 kPa and a temperature of 300 °C. At this
temperature, it fully decomposes.
(a) Write a balanced equation for the decomposition of calcium nitrate.
(b) Determine the number of moles of calcium nitrate used.
(c) Calculate the volume of the nitrogen dioxide and oxygen gas evolved and thus, the total
rolume of gases evolved under the conditions of the experiment.
Page
2 2
+
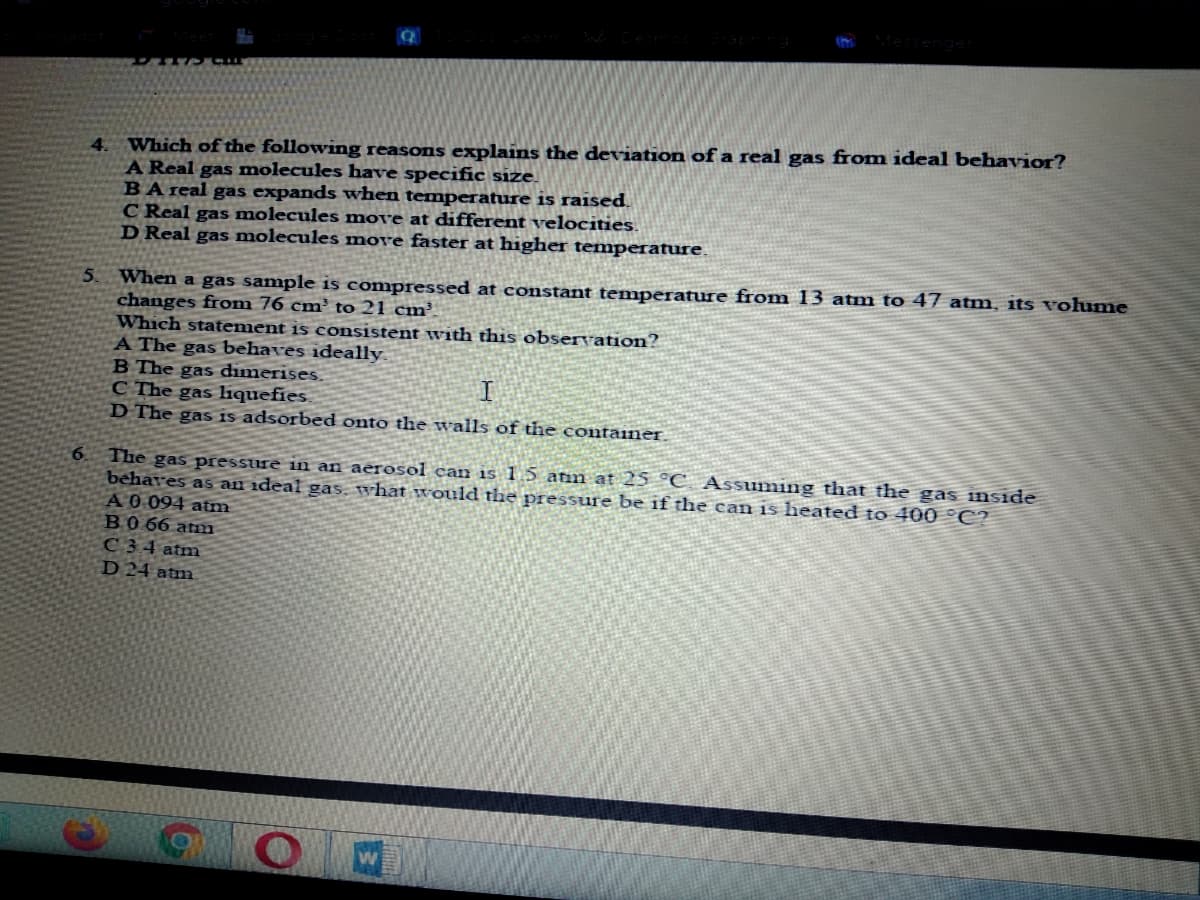
Transcribed Image Text:4. Which of the following reasons explains the deviation of a real gas from ideal behavior?
A Real gas molecules have specific size.
BA real gas expands when temperature is raised.
C Real gas molecules move at different velocities.
D Real gas molecules move faster at higher temperature.
5. When a gas sample is compressed at constant temperature from 13 atm to 47 atm, its vohume
changes from 76 cm² to 21 cm³
Which statement is consistent with this observation?
A The gas behaves ideally.
B The gas dimerises.
C The gas liquefies.
D The gas is adsorbed onto the walls of the containe.
6.
The gas pressure in an aèrosol can is 1 5 atin at 25 °C Assuming that the gas inside
behaves as an ideal gas, what would the pressure be if tlhe can is heated to 400 °C?
A0 094 atm
BO 66 atm
C34 atm
D 24 atm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning