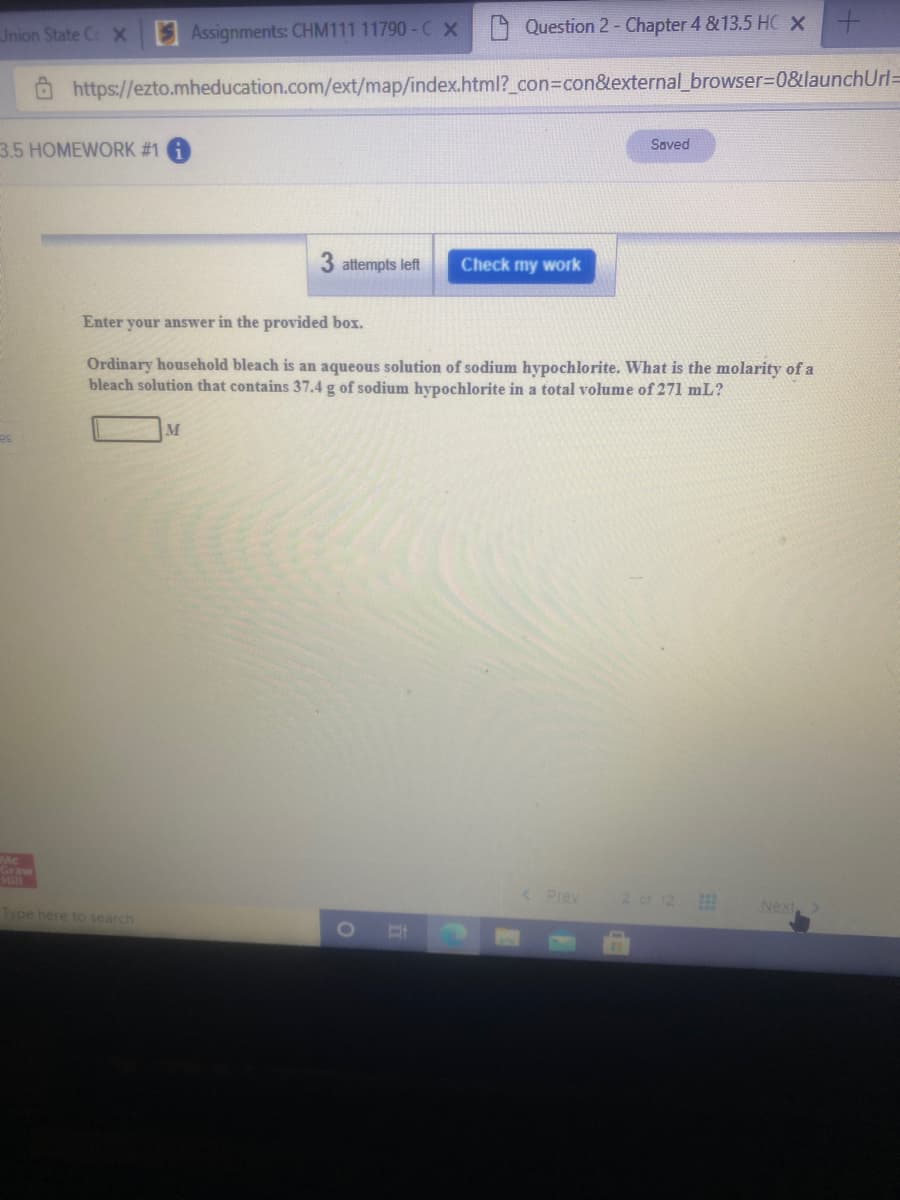
Ordinary household bleach is an aqueous solution of sodium hypochlorite. What is the molarity of a bleach solution that contains 37.4 g of sodium hypochlorite in a total volume of 271 mL? M
Ordinary household bleach is an aqueous solution of sodium hypochlorite. What is the molarity of a bleach solution that contains 37.4 g of sodium hypochlorite in a total volume of 271 mL? M
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.9QAP
Related questions
Question
Transcribed Image Text:Jnion State CX
SAssignments: CHM111 11790- C X
Question 2- Chapter 4 &13.5 HC x -
O https://ezto.mheducation.com/ext/map/index.html?_con3Dcon&external_browser%3D0&ulaunchUrl=
Saved
3.5 HOMEWORK #1
3 attempts left
Check my work
Enter your answer in the provided box.
Ordinary household bleach is an aqueous solution of sodium hypochlorite. What is the molarity of a
bleach solution that contains 37.4 g of sodium hypochlorite in a total volume of 271 mL?
Mc
Graw
< Prev
2 or 12
Type here to search
Next
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

