Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter23: Amines
Section23.9: Hofmann Elimination
Problem 23.14P
Related questions
Question
Transcribed Image Text:9::01
令all
اسئلة کيمياء عض. . .
>
Organic Chemistry
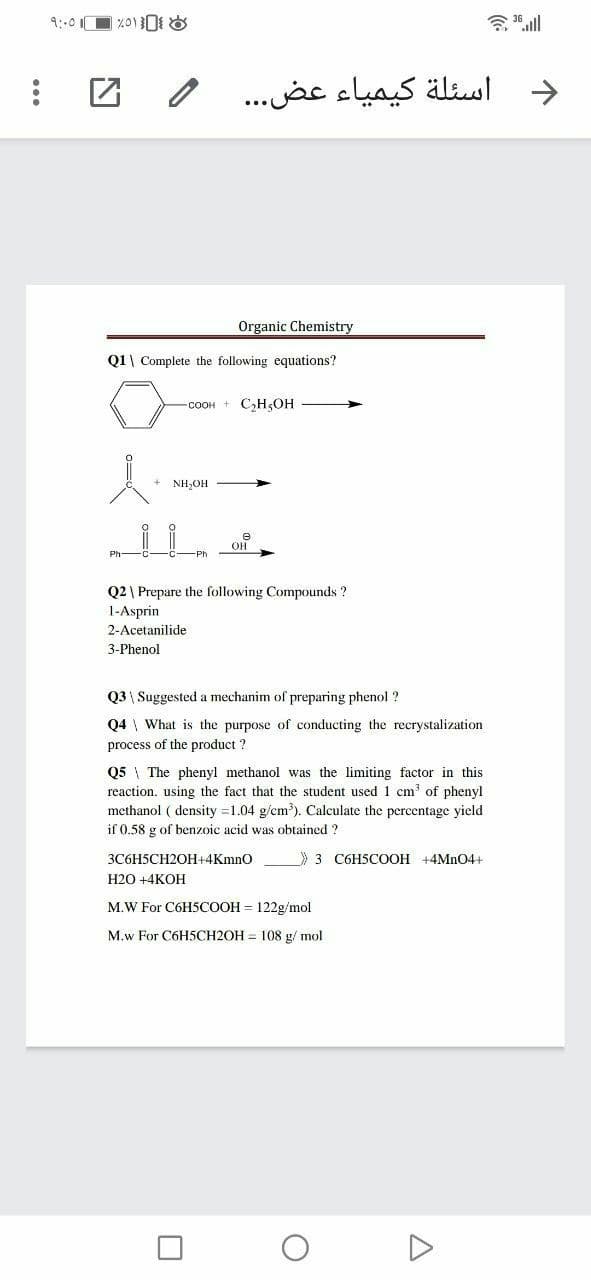
Q1| Complete the following equations?
-COOH + C,H,OH
NH,OH
он
Q2 | Prepare the following Compounds ?
1-Asprin
2-Acetanilide
3-Phenol
Q3 \ Suggested a mechanim of preparing phenol ?
Q4 \ What is the purpose of conducting the recrystalization
process of the product ?
Q5 \ The phenyl methanol was the limiting factor in this
reaction. using the fact that the student used 1 cm of phenyl
methanol ( density =1.04 g/cm). Calculate the percentage yield
if 0.58 g of benzoic acid was obtained ?
3C6H5CH2OH+4Kmno
3 C6H5COOH +4MN04+
Н2О +4КОН
M.W For C6H5COOH = 122g/mol
M.w For C6H5CH2OH = 108 g/ mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning