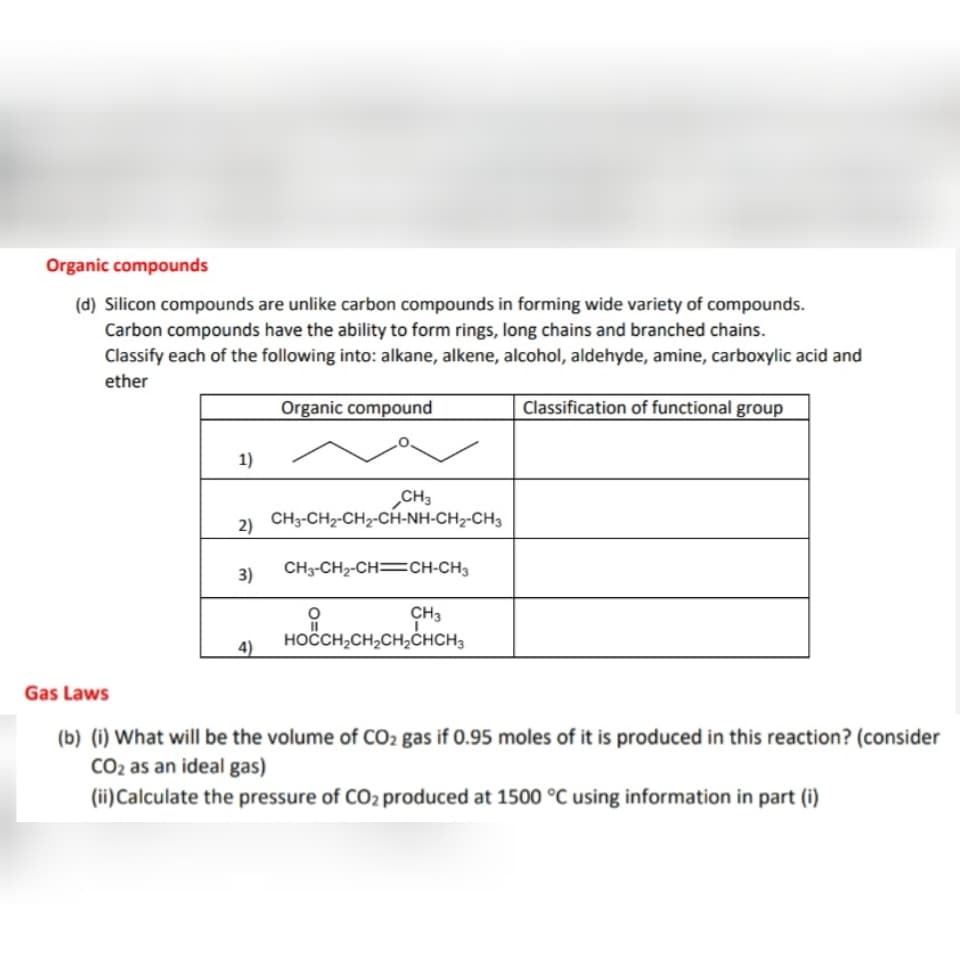
Organic compounds (d) Silicon compounds are unlike carbon compounds in forming wide variety of compounds. Carbon compounds have the ability to form rings, long chains and branched chains. Classify each of the following into: alkane, alkene, alcohol, aldehyde, amine, carboxylic acid and ether Organic compound Classification of functional group 1) CH3 CH3-CH2-CH2-CH-NH-CH2-CH3 2) 3) CH3-CH2-CH=CH-CH3 CH3 HOČCH,CH,CH,CHCH, 4)
Organic compounds (d) Silicon compounds are unlike carbon compounds in forming wide variety of compounds. Carbon compounds have the ability to form rings, long chains and branched chains. Classify each of the following into: alkane, alkene, alcohol, aldehyde, amine, carboxylic acid and ether Organic compound Classification of functional group 1) CH3 CH3-CH2-CH2-CH-NH-CH2-CH3 2) 3) CH3-CH2-CH=CH-CH3 CH3 HOČCH,CH,CH,CHCH, 4)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section: Chapter Questions
Problem 10.BCP
Related questions
Question
Transcribed Image Text:Organic compounds
(d) Silicon compounds are unlike carbon compounds in forming wide variety of compounds.
Carbon compounds have the ability to form rings, long chains and branched chains.
Classify each of the following into: alkane, alkene, alcohol, aldehyde, amine, carboxylic acid and
ether
Organic compound
|Classification of functional group
1)
CH3
CH3-CH2-CH2-CH-NH-CH2-CH3
2)
3)
CH3-CH2-CH=CH-CH3
CH3
HOČCH,CH,CH2ĆHCH3
4)
Gas Laws
(b) (i) What will be the volume of CO2 gas if 0.95 moles of it is produced in this reaction? (consider
CO2 as an ideal gas)
(ii)Calculate the pressure of CO2 produced at 1500 °C using information in part (i)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning