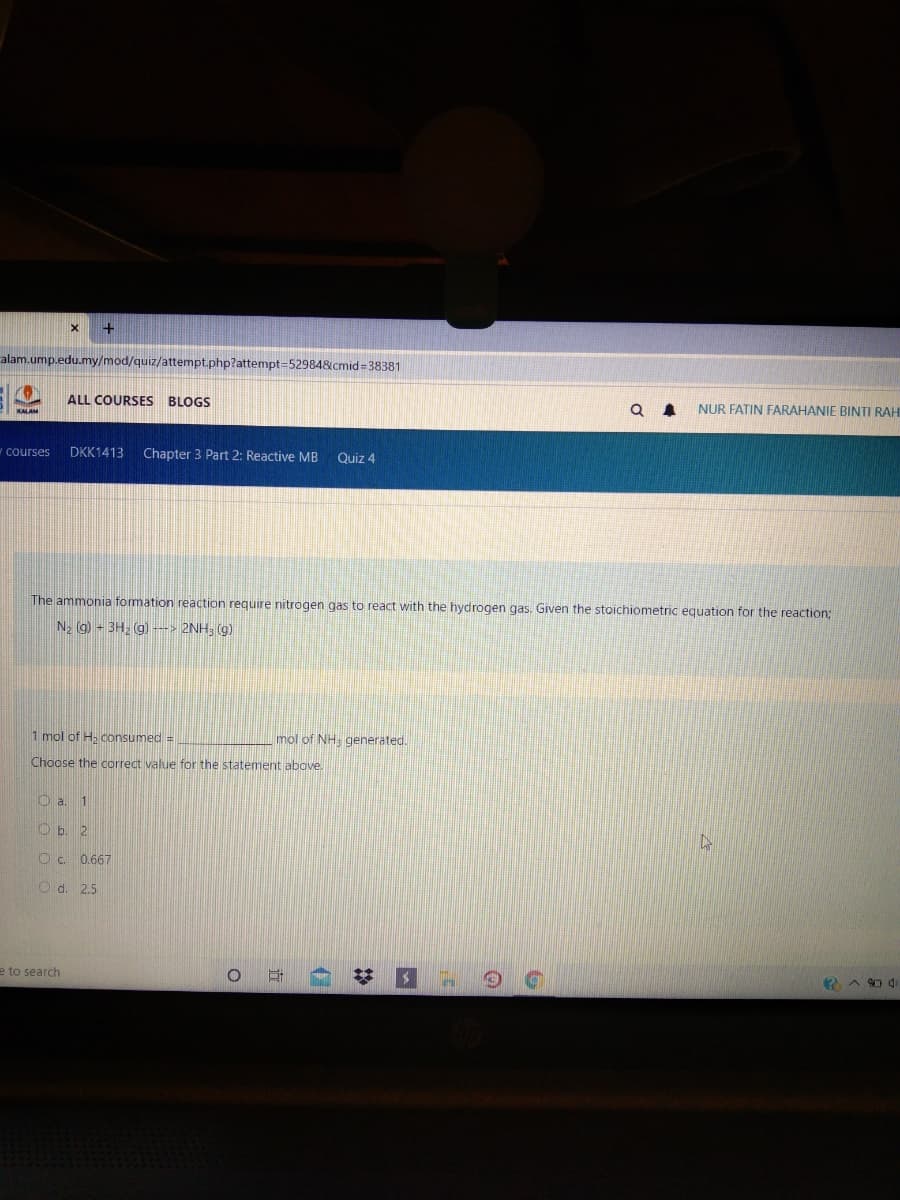
ourses DKK1413 Chapter 3 Part 2: Reactive MB Quiz 4 The ammonia formation reaction require nitrogen gas to react with the hydrogen gas. Given the stoichiometric equation for the reaction; N2 (g) - 3H; (g) --> 2NH3 (g) 1 mol of H, consumed = mol of NH, generated. Choose the correct value for the statement above. O a. 1 O b. 2 Oc 0.667
ourses DKK1413 Chapter 3 Part 2: Reactive MB Quiz 4 The ammonia formation reaction require nitrogen gas to react with the hydrogen gas. Given the stoichiometric equation for the reaction; N2 (g) - 3H; (g) --> 2NH3 (g) 1 mol of H, consumed = mol of NH, generated. Choose the correct value for the statement above. O a. 1 O b. 2 Oc 0.667
Chapter11: Solving Equilibrium Problems For Complex Systems
Section: Chapter Questions
Problem 11.3QAP
Related questions
Question
Transcribed Image Text:alam.ump.edu.my/mod/quiz/attempt.php?attempt-52984&cmid 38381
ALL COURSES BLOGS
NUR FATIN FARAHANIE BINTI RAH
courses
DKK1413
Chapter 3 Part 2: Reactive MB
Quiz 4
The ammonia formation reaction require nitrogen gas to react with the hydrogen gas. Given the stoichiometric equation for the reaction;
N2 g) - 3H, (g) ---> 2NH; (g)
1 mol of H. consumed =
mol of NH. generated.
Choose the correct value for the statement above.
O a. 1
O b. 2
O c. 0.667
O d. 2.5
e to search
立
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning