Ox agent: Red agent: 1. Fe3* aq) + Sn2*(aq) → Fe2*taq) + Sn*(a (aq) HNO2(aq) + MnO4¯(aq) → Mn2*(aq) + NO3¯(aq) Ox agent: Red agent: 2. SO3²"(aq) + Mn04"(aq) → SO42 (aq) + MnO2(aq) (not peroxide) Ox agent: Red agent: 3.
Ox agent: Red agent: 1. Fe3* aq) + Sn2*(aq) → Fe2*taq) + Sn*(a (aq) HNO2(aq) + MnO4¯(aq) → Mn2*(aq) + NO3¯(aq) Ox agent: Red agent: 2. SO3²"(aq) + Mn04"(aq) → SO42 (aq) + MnO2(aq) (not peroxide) Ox agent: Red agent: 3.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.98PAE
Related questions
Question
please
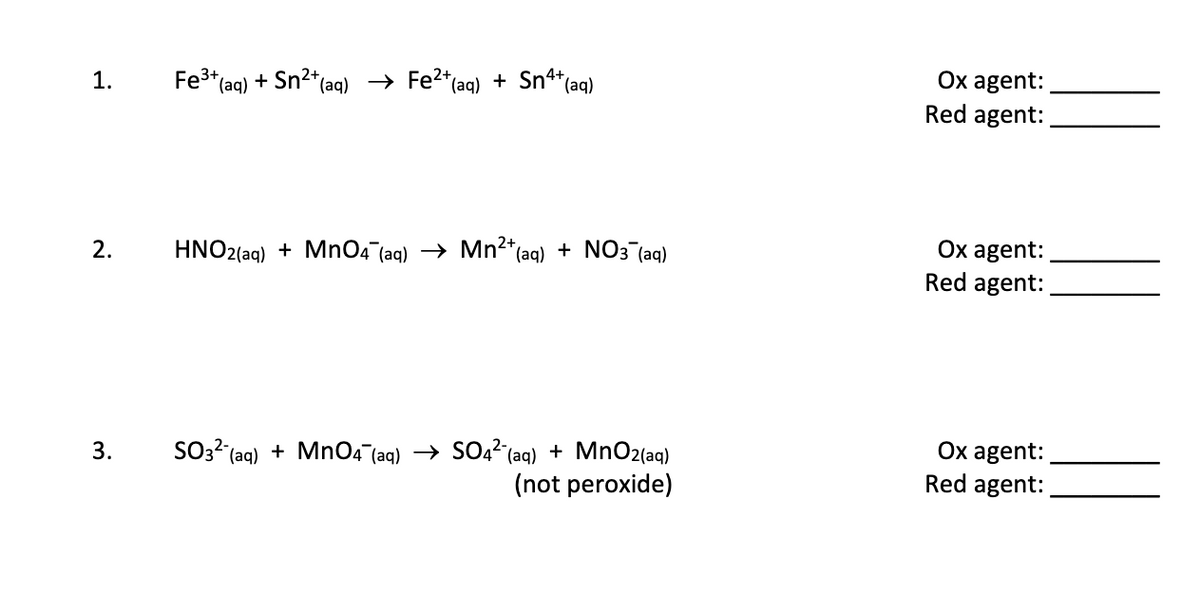
Transcribed Image Text:Ox agent:
1.
Fe3* (aq) + Sn2*(aq) → Fe2*(aq) + Sn*(aq)
Red agent:
Ox agent:
Red agent:
2.
HNO2(aq) + MnO4 (aq) → Mn²*(aq) + NO3 (aq)
SO3? (aq) + MnO4 (aq) → SO22 (aq) + MnO2(aq)
(not peroxide)
Ox agent:
Red agent:
3.
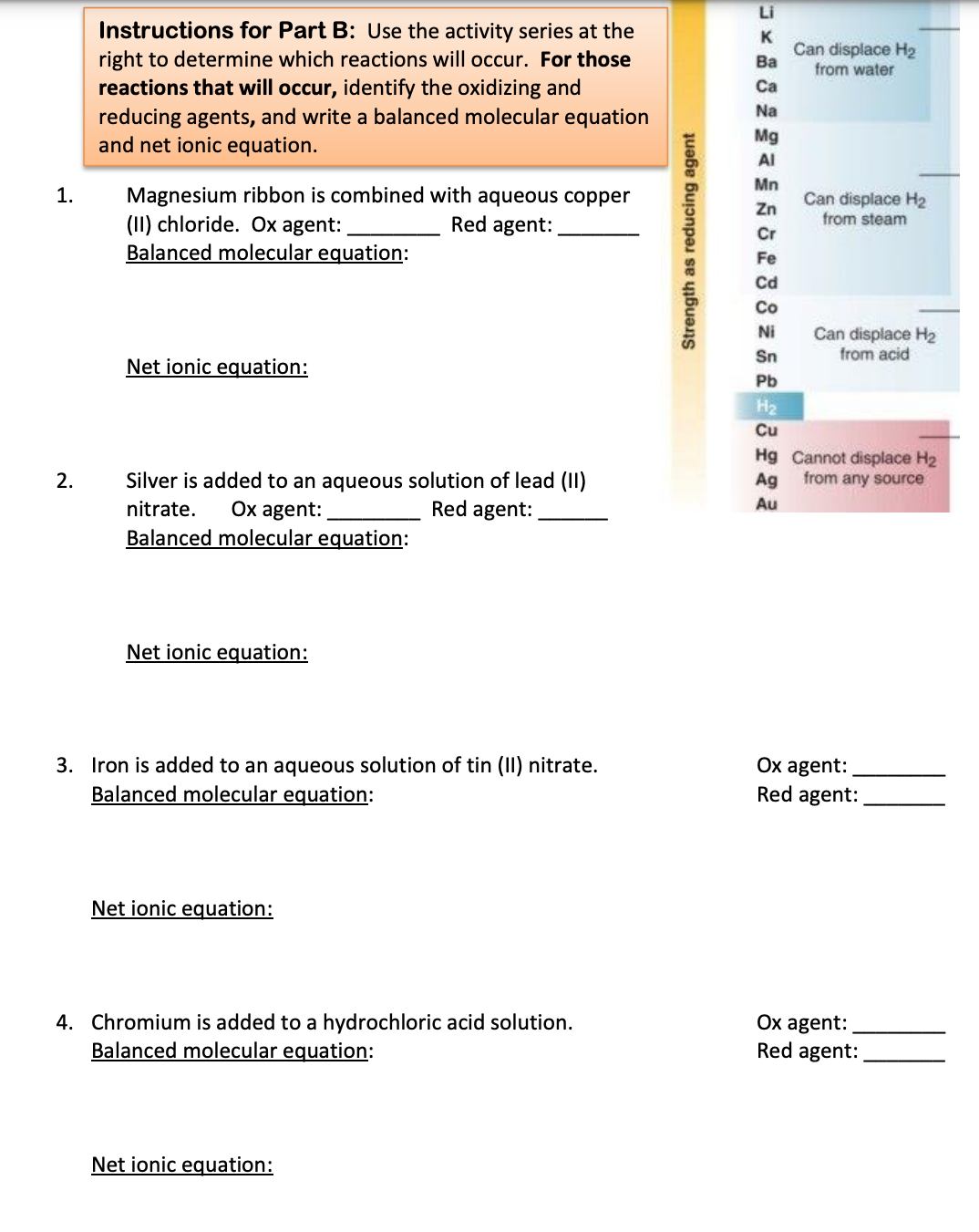
Transcribed Image Text:Li
Instructions for Part B: Use the activity series at the
right to determine which reactions will occur. For those
reactions that will occur, identify the oxidizing and
reducing agents, and write a balanced molecular equation
and net ionic equation.
K
Can displace H2
Ba
from water
Ca
Na
Mg
AI
Mn
Magnesium ribbon is combined with aqueous copper
(II) chloride. Ox agent:
Balanced molecular equation:
1.
Can displace H2
Zn
Red agent:
from steam
Cr
Fe
Cd
Co
Can displace H2
from acid
Ni
Sn
Net ionic equation:
Pb
H2
Cu
Hg Cannot displace H2
Ag from any source
Silver is added to an aqueous solution of lead (II)
Red agent:
2.
Ox agent:
Balanced molecular equation:
nitrate.
Au
Net ionic equation:
3. Iron is added to an aqueous solution of tin (II) nitrate.
Balanced molecular equation:
Ох agent:
Red agent:
Net ionic equation:
4. Chromium is added to a hydrochloric acid solution.
Balanced molecular equation:
Ox agent:
Red agent:
Net ionic equation:
Strength as reducing agent
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning