Oxidation & Reduction: 61. Consider the reaction Which statement is true for the reaction? Fe3+ is oxidized. (A) (C) Fe³+ is reduced. 62. In the chemical reaction, (A) (C) 2Fe³+ (aq) + 21 (aq) →2Fe2+ (aq) + I₂(aq) (B) (D) Fe³+ increases in oxidation number. I- is reduced. Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s), metallic zinc is the reducing agent. copper ion is oxidized. (B) (D) metallic zinc in reduced. sulfate ion is the oxidizing agent.
Oxidation & Reduction: 61. Consider the reaction Which statement is true for the reaction? Fe3+ is oxidized. (A) (C) Fe³+ is reduced. 62. In the chemical reaction, (A) (C) 2Fe³+ (aq) + 21 (aq) →2Fe2+ (aq) + I₂(aq) (B) (D) Fe³+ increases in oxidation number. I- is reduced. Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s), metallic zinc is the reducing agent. copper ion is oxidized. (B) (D) metallic zinc in reduced. sulfate ion is the oxidizing agent.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 61E: Consider only the species (at standard conditions) Na+, Cl, Ag+, Ag, Zn2+, Zn, Pb in answering the...
Related questions
Question
I need help with questions 61-62 if possible? Could you also explain which option is the correct answer for each question?
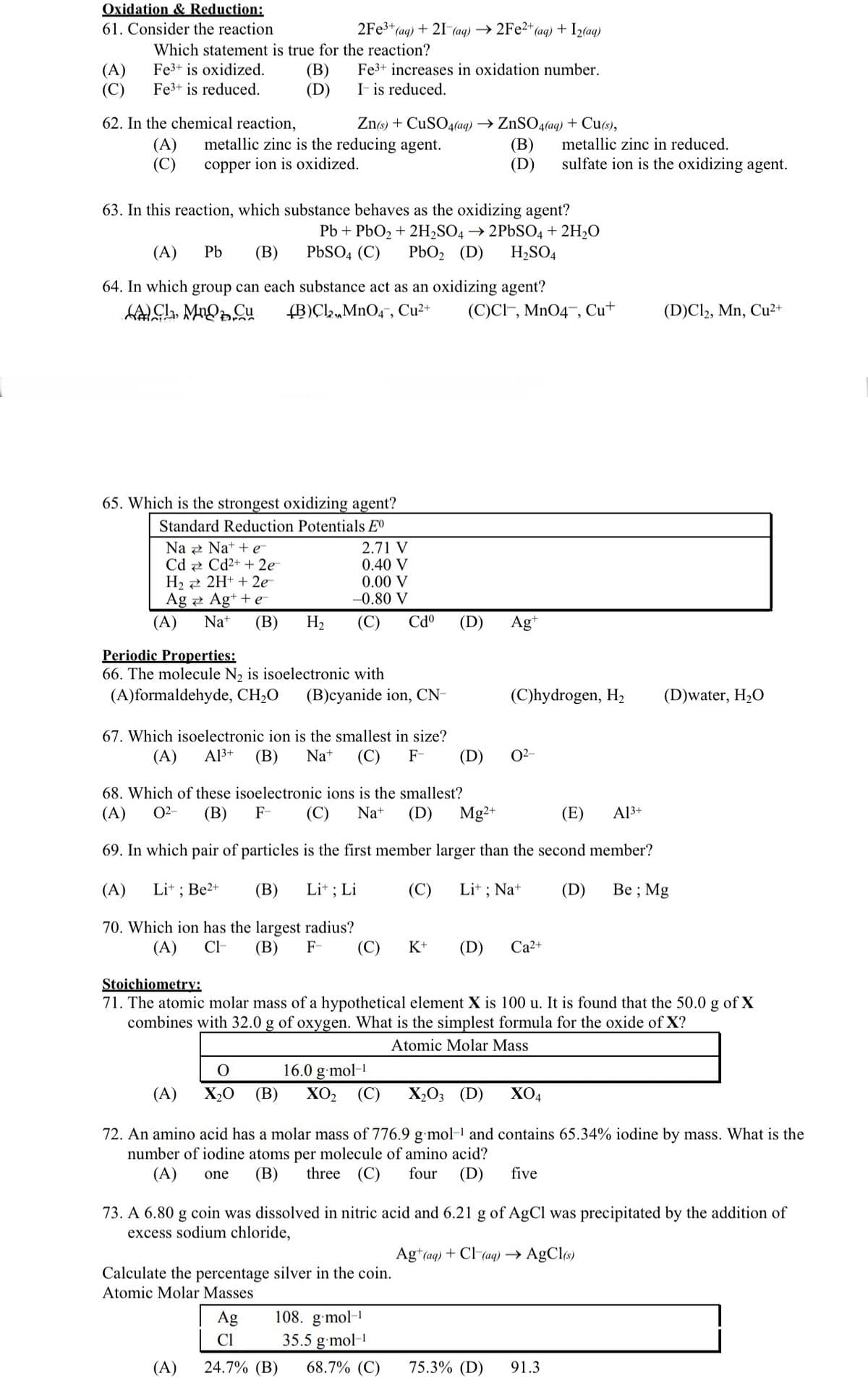
Transcribed Image Text:Oxidation & Reduction:
61. Consider the reaction
(A)
(C)
Which statement is true for the reaction?
Fe3+ is oxidized.
Fe3+ is reduced.
62. In the chemical reaction,
(A)
(C)
(B)
(D)
2Fe³+ (aq) + 21 (aq) →2Fe2+(
Na
Cd
H₂
Ag
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s),
metallic zinc is the reducing agent.
copper ion is oxidized.
(A)
Fe3+ increases in oxidation number.
It is reduced.
63. In this reaction, which substance behaves as the oxidizing agent?
Pb + PbO₂ + 2H₂SO4 → 2PbSO4 + 2H₂O
(A) Pb (B) PbSO4 (C) PbO₂ (D) H₂SO4
65. Which is the strongest oxidizing agent?
Standard Reduction Potentials Eº
64. In which group can each substance act as an oxidizing agent?
ACL, MnO Cu (B)Cl,MnO4, Cu²+ (C)CI, MnO4, Cu+
Nat+e¯¯
Cd²+ + 2e
2H+ + 2e-
Ag+ + e
Na+ (B) H₂
2.71 V
0.40 V
0.00 V
-0.80 V
Periodic Properties:
66. The molecule N₂ is isoelectronic with
(A)formaldehyde, CH₂O (B)cyanide ion, CN-
+ (aq) + I₂(aq)
Cdº
67. Which isoelectronic ion is the smallest in size?
(A) A1³+ (B) Na+ (C) F-
Lit ; Li
(B)
(D)
(D)
(D)
Calculate the percentage silver in the coin.
Atomic Molar Masses
Agt
(C)hydrogen, H₂
0²-
metallic zinc in reduced.
sulfate ion is the oxidizing agent.
68. Which of these isoelectronic ions is the smallest?
(E) A13+
(A) 0²- (B) F- (C) Na+ (D) Mg2+
69. In which pair of particles is the first member larger than the second member?
(A) Li+; Be2+
(B)
(C) Lit; Na+
(D)
Be; Mg
70. Which ion has the largest radius?
(A) CI- (B) F-
(C)
K+ (D) Ca²+
O
16.0 g.mol-1
X₂0 (B) XO₂ (C) X₂03 (D) XO4
Stoichiometry:
71. The atomic molar mass of a hypothetical element X is 100 u. It is found that the 50.0 g of X
combines with 32.0 g of oxygen. What is the simplest formula for the oxide of X?
Atomic Molar Mass
(D)Cl2, Mn, Cu²+
(D)water, H₂O
(A)
72. An amino acid has a molar mass of 776.9 g mol-¹ and contains 65.34% iodine by mass. What is the
number of iodine atoms per molecule of amino acid?
(A) one (B) three (C) four (D) five
73. A 6.80 g coin was dissolved in nitric acid and 6.21 g of AgCl was precipitated by the addition of
excess sodium chloride,
Ag+ (aq) + Cl(aq) → AgCl(s)
Ag
108. g.mol-1
Cl
35.5 g.mol-¹
(A) 24.7% (B) 68.7% (C) 75.3% (D) 91.3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning