P = P = P = Al N = N = N=- E = E = Bohr Diagram o Bohr Diagram eod Bohr Diagram Al F Lewis Structure Lewis Structure Lewis Structure P = P = P = Ar Si Na N = N = N =_ E = E = E = Bohr Diagram Bohr Diagram vio Bohr Diagrami Lewis Structure Ar Lewis Structure Si Lewis Structure Na P = P = P = Be CI N = N = N = E = E = E = Bohr Diagramg Bohr Diagram Bohr Diagram c Lewis Structure Be CI Lewis Structure Lewis Structure
P = P = P = Al N = N = N=- E = E = Bohr Diagram o Bohr Diagram eod Bohr Diagram Al F Lewis Structure Lewis Structure Lewis Structure P = P = P = Ar Si Na N = N = N =_ E = E = E = Bohr Diagram Bohr Diagram vio Bohr Diagrami Lewis Structure Ar Lewis Structure Si Lewis Structure Na P = P = P = Be CI N = N = N = E = E = E = Bohr Diagramg Bohr Diagram Bohr Diagram c Lewis Structure Be CI Lewis Structure Lewis Structure
An Introduction to Physical Science
14th Edition
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Chapter9: Atomic Physics
Section: Chapter Questions
Problem HM
Related questions
Question
100%
I need help with this worksheet
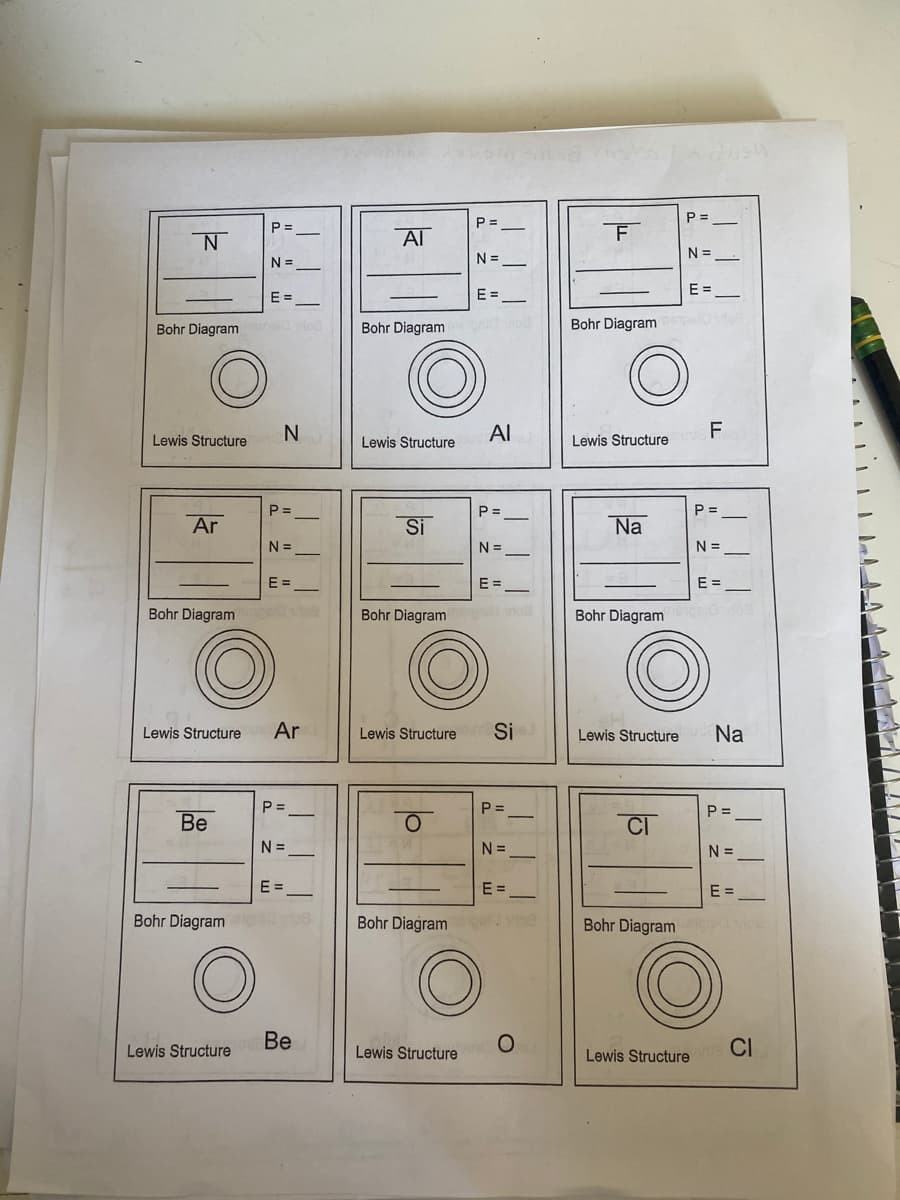
Transcribed Image Text:P =
P =
P =
Al
N =
N =
N =
E =
E =
E =
Bohr Diagram
Bohr Diagram eod
Bohr Diagram
Al
F
Lewis Structure
Lewis Structure
Lewis Structure
P=
P =
P =
Ar
Si
Na
N=
N =
N =_
E =
E =
E =
Bohr Diagram
Bohr Diagram
Bohr Diagram s
Lewis Structure
Ar
Lewis Structure
Si
Lewis Structure
Na
P =
P =
P =
Be
CI
N =
N =
N =
E =
E =
E =
Bohr Diagramig o9
Bohr Diagram
Bohr Diagram o
Ве
CI
Lewis Structure
Lewis Structure
Lewis Structure
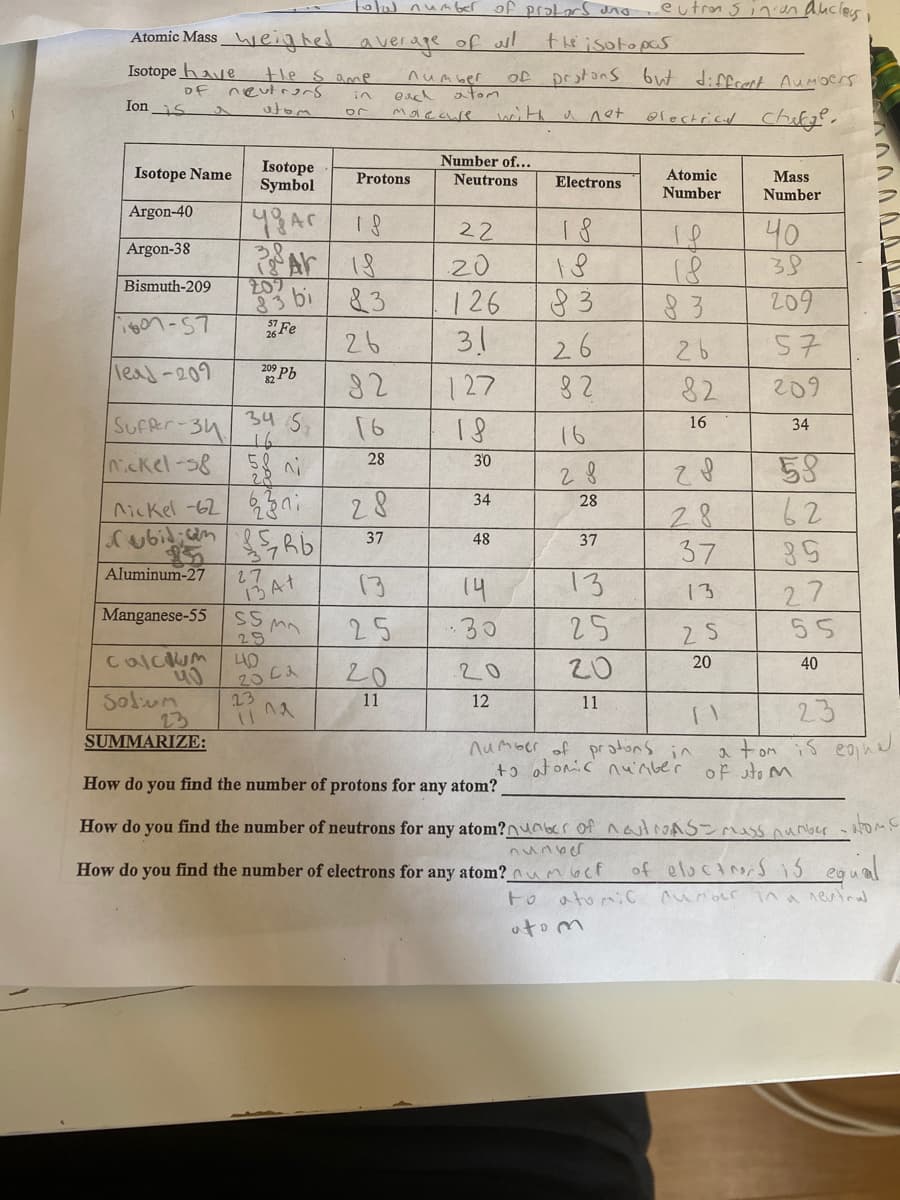
Transcribed Image Text:olul num ber of protLars ano
eutron sinien aucles ,
Atomic Mass_eighel average of ul
the isotopas
Isotope have
the s ame
neutrors
atom
number
protons but diffrect Aumoers
of
Df
eacl ator
in
Ion s
with
electrical chilg",
a net
Number of...
Isotope
Symbol
Isotope Name
Atomic
Number
Mass
Number
Protons
Neutrons
Electrons
Argon-40
1 8
18
83
18
18
83
31
40
38
22
to
18
83
Argon-38
38
20
207
83 bi
Bismuth-209
126
209
Fe
26
26
26
57
leas-209
Pb
127
8 2
82
209
SuFRr-34
Nckel-8
34
16
16
18
34
16
58
62
28
30
2.
28
nicKel -62 3ni
Subidicam
34
28
28
28
37
37
48
37
Aluminum-27
27
14
13
27
13
Manganese-55
SS mn
25
25
.30
2 5
55
25
L40
20 C2
Calcum
20
20
40
Oh
23
20
20
Solum
11
12
11
23
23
SUMMARIZE:
number of protons in
+ɔ at onic nu'nbe'r
a tom is eond
of sto m
How do you find the number of protons for any atom?
How do you find the number of neutrons for any atom?nuabr of nautroAS-mass nunber - atoMS
of elociners is egual
to atomiC unocr ina neuinul
How do you find the number of electrons for any atom? numoct
oto m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

An Introduction to Physical Science
Physics
ISBN:
9781305079137
Author:
James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:
Cengage Learning

Glencoe Physics: Principles and Problems, Student…
Physics
ISBN:
9780078807213
Author:
Paul W. Zitzewitz
Publisher:
Glencoe/McGraw-Hill

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Modern Physics
Physics
ISBN:
9781111794378
Author:
Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax