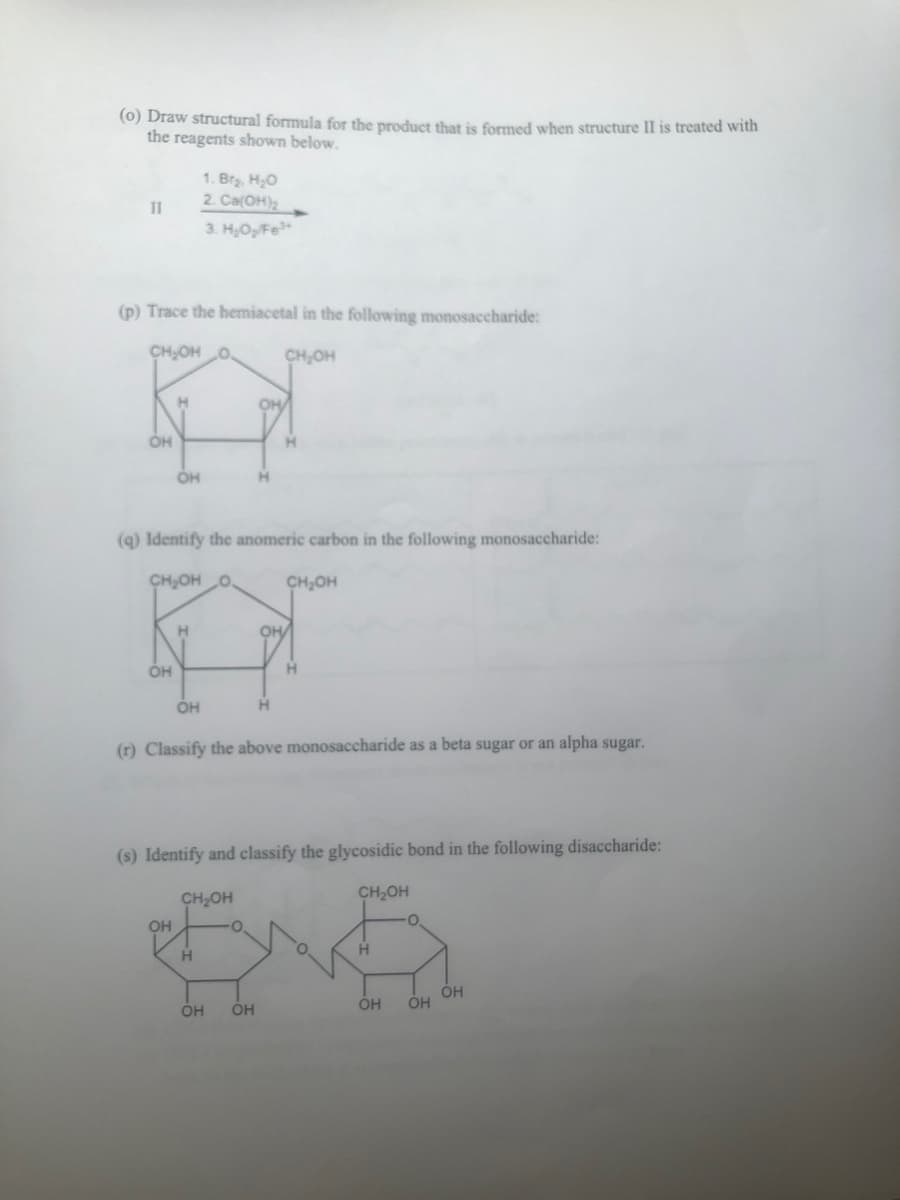
(p) Trace the hemiacetal in the following monosaccharide: CH₂OH O CH₂OH OH OH OH (q) Identify the anomeric carbon in the following monosaccharide: CH OH O H OH OH CH₂OH OH (r) Classify the above monosaccharide as a beta sugar or an alpha sugar. H Н (s) Identify and classify the glycosidic bond in the following disaccharide: ОН ОН CH₂OH OH CH₂OH ОН OH ОН
Carbohydrates
Carbohydrates are the organic compounds that are obtained in foods and living matters in the shape of sugars, cellulose, and starch. The general formula of carbohydrates is Cn(H2O)2. The ratio of H and O present in carbohydrates is identical to water.
Starch
Starch is a polysaccharide carbohydrate that belongs to the category of polysaccharide carbohydrates.
Mutarotation
The rotation of a particular structure of the chiral compound because of the epimerization is called mutarotation. It is the repercussion of the ring chain tautomerism. In terms of glucose, this can be defined as the modification in the equilibrium of the α- and β- glucose anomers upon its dissolution in the solvent water. This process is usually seen in the chemistry of carbohydrates.
L Sugar
A chemical compound that is represented with a molecular formula C6H12O6 is called L-(-) sugar. At the carbon’s 5th position, the hydroxyl group is placed to the compound’s left and therefore the sugar is represented as L(-)-sugar. It is capable of rotating the polarized light’s plane in the direction anticlockwise. L isomers are one of the 2 isomers formed by the configurational stereochemistry of the carbohydrates.

Step by step
Solved in 3 steps with 3 images







