P17D.5* Methane is a by-product of a number of natural processes (such as digestion of cellulose in ruminant animals, and anaerobic decomposition of organic waste matter), and industrial processes (such as food production and fossil fuel use). Reaction with the hydroxyl radical OH is the main path by which CH, is removed from the lower atmosphere. T. Gierczak et al. (J. Phys. Chem. A 101, 3125 (1997)) measured the rate constants for the elementary bimolecular gas-phase reaction of methane with the hydroxyl radical over a range of temperatures of importance to atmospheric chemistry. Deduce the Arrhenius parameters A and E, from the following measurements: T/K 295 223 218 213 206 200 195 kJ(10ʻdm'mol's) 3.55 0.494 0.452 0.379 0.295 0.241 0.217
P17D.5* Methane is a by-product of a number of natural processes (such as digestion of cellulose in ruminant animals, and anaerobic decomposition of organic waste matter), and industrial processes (such as food production and fossil fuel use). Reaction with the hydroxyl radical OH is the main path by which CH, is removed from the lower atmosphere. T. Gierczak et al. (J. Phys. Chem. A 101, 3125 (1997)) measured the rate constants for the elementary bimolecular gas-phase reaction of methane with the hydroxyl radical over a range of temperatures of importance to atmospheric chemistry. Deduce the Arrhenius parameters A and E, from the following measurements: T/K 295 223 218 213 206 200 195 kJ(10ʻdm'mol's) 3.55 0.494 0.452 0.379 0.295 0.241 0.217
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter21: Benzene And The Concept Of Aromaticity
Section: Chapter Questions
Problem 21.44P
Related questions
Question
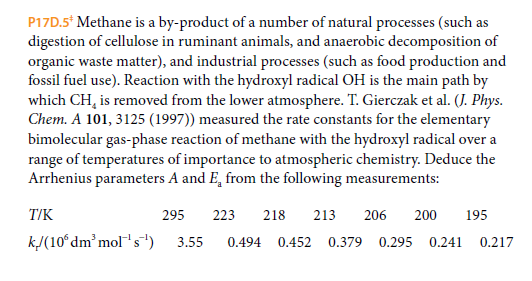
Transcribed Image Text:P17D.5* Methane is a by-product of a number of natural processes (such as
digestion of cellulose in ruminant animals, and anaerobic decomposition of
organic waste matter), and industrial processes (such as food production and
fossil fuel use). Reaction with the hydroxyl radical OH is the main path by
which CH, is removed from the lower atmosphere. T. Gierczak et al. (J. Phys.
Chem. A 101, 3125 (1997)) measured the rate constants for the elementary
bimolecular gas-phase reaction of methane with the hydroxyl radical over a
range of temperatures of importance to atmospheric chemistry. Deduce the
Arrhenius parameters A and E, from the following measurements:
T/K
295
223
218
213
206
200
195
kJ(10ʻdm'mol's)
3.55
0.494 0.452
0.379 0.295 0.241
0.217
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
