Ethyl butanoate, CH;CH;CH;CO,CH;CH3, is one of the many organic compounds isolated from mangoes. Which hydrogen is most readily removed when ethyl butanoate is treated with base? Propose a reason for your choice, and using the data listed below, estimate its pK,. Compound pK, CH,CONH, 16 CH,CHO 17 (CH,),COH 18 (CH3,C=O 19.2 CH,CO,CH,CH, 24.5 HC=CH 25 CH,C=N 25 CHCI, 25 CH,CON(CH,)2 30 The most readily removed type of hydrogen is on the: O CH; bonded to C=0. CH2 of the ethyl group. CH3 of the propyl group. CH3 of the ethyl group. This type of hydrogen is most readily removed because: O The conjugate base is stabilized by resonance. The conjugate base is stabilized by inductance. O Loss of the proton deceases steric hindrance around the C=0. The conjugate base is stabilized by the high electronegativity of O. The estimated pK, is
Ethyl butanoate, CH;CH;CH;CO,CH;CH3, is one of the many organic compounds isolated from mangoes. Which hydrogen is most readily removed when ethyl butanoate is treated with base? Propose a reason for your choice, and using the data listed below, estimate its pK,. Compound pK, CH,CONH, 16 CH,CHO 17 (CH,),COH 18 (CH3,C=O 19.2 CH,CO,CH,CH, 24.5 HC=CH 25 CH,C=N 25 CHCI, 25 CH,CON(CH,)2 30 The most readily removed type of hydrogen is on the: O CH; bonded to C=0. CH2 of the ethyl group. CH3 of the propyl group. CH3 of the ethyl group. This type of hydrogen is most readily removed because: O The conjugate base is stabilized by resonance. The conjugate base is stabilized by inductance. O Loss of the proton deceases steric hindrance around the C=0. The conjugate base is stabilized by the high electronegativity of O. The estimated pK, is
Chapter27: Biomolecules: Lipids
Section27.SE: Something Extra
Problem 47AP: Cembrene, C20H32, is a diterpenoid hydrocarbon isolated from pine resin. Cembrene has a UV...
Related questions
Question
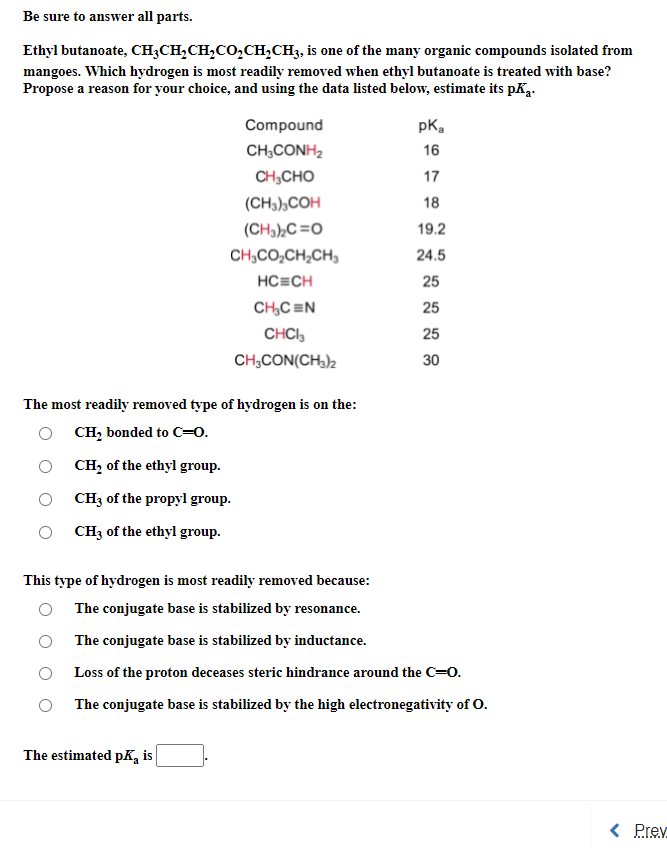
Transcribed Image Text:Be sure to answer all parts.
Ethyl butanoate, CH;CH,CH,CO,CH,CH3, is one of the many organic compounds isolated from
mangoes. Which hydrogen is most readily removed when ethyl butanoate is treated with base?
Propose a reason for your choice, and using the data listed below, estimate its pK2.
Compound
pK,
CH;CONH2
16
CH,CHO
17
(CH3),COH
18
(CH3),C=O
19.2
CH,CO,CH,CH,
24.5
HC=CH
25
CH,C =N
25
CHCI,
25
CH,CON(CH3)2
30
The most readily removed type of hydrogen is on the:
O CH, bonded to C=0.
CH, of the ethyl group.
CH3 of the propyl group.
CH3 of the ethyl group.
This type of hydrogen is most readily removed because:
The conjugate base is stabilized by resonance.
The conjugate base is stabilized by inductance.
Loss of the proton deceases steric hindrance around the C=0.
The conjugate base is stabilized by the high electronegativity of O.
The estimated pK, is
< Prev
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning