P6B.8 The dissociation of I,(g) can be monitored by measuring the total pressure, and three sets of results are as follows: T/K 973 1073 1173 100p/atm 6.244 6.500 9.181 10ʻn. 2.4709 2.4555 2.4366 where n, is the amount of I, molecules introduced into a container of volume 342.68 cm'. Calculate the equilibrium constants of the dissociation and the standard enthalpy of dissociation assuming it to be constant over the range of temperatures.
P6B.8 The dissociation of I,(g) can be monitored by measuring the total pressure, and three sets of results are as follows: T/K 973 1073 1173 100p/atm 6.244 6.500 9.181 10ʻn. 2.4709 2.4555 2.4366 where n, is the amount of I, molecules introduced into a container of volume 342.68 cm'. Calculate the equilibrium constants of the dissociation and the standard enthalpy of dissociation assuming it to be constant over the range of temperatures.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.67PAE
Related questions
Question
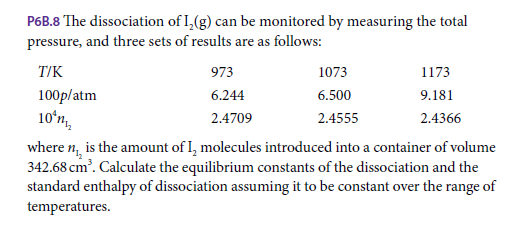
Transcribed Image Text:P6B.8 The dissociation of I,(g) can be monitored by measuring the total
pressure, and three sets of results are as follows:
T/K
973
1073
1173
100p/atm
6.244
6.500
9.181
10ʻn.
2.4709
2.4555
2.4366
where n, is the amount of I, molecules introduced into a container of volume
342.68 cm'. Calculate the equilibrium constants of the dissociation and the
standard enthalpy of dissociation assuming it to be constant over the range of
temperatures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 8 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning