Part A Calculate the pH of a buffer solution composed of 0.080 mol · L-1 chloroacetic acid (CH2CICOOH) and 0.025 mol · L- sodium chloroacetate (CH2C1COONa) by using the equilibrium method. The Ka for CH2CICOOH is 1.4 × 10-3. Express your answer to two decimal places. AZ¢ pH = Submit Request Answer Part B Calculate the pH of a buffer solution composed of 0.080 mol · L-1 chloroacetic acid (CH2 CICOOH) and 0.025 mol · L- sodium chloroacetate (CH2 cICOONa) by using the Henderson-Hasselbalch equation. The Ka for CH2C1COOH is 1.4 × 10-3, Express your answer to two decimal places. ΑΣφ ? pH = Submit Request Answer
Part A Calculate the pH of a buffer solution composed of 0.080 mol · L-1 chloroacetic acid (CH2CICOOH) and 0.025 mol · L- sodium chloroacetate (CH2C1COONa) by using the equilibrium method. The Ka for CH2CICOOH is 1.4 × 10-3. Express your answer to two decimal places. AZ¢ pH = Submit Request Answer Part B Calculate the pH of a buffer solution composed of 0.080 mol · L-1 chloroacetic acid (CH2 CICOOH) and 0.025 mol · L- sodium chloroacetate (CH2 cICOONa) by using the Henderson-Hasselbalch equation. The Ka for CH2C1COOH is 1.4 × 10-3, Express your answer to two decimal places. ΑΣφ ? pH = Submit Request Answer
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter14: Equilibria In Acid-base Solutions
Section: Chapter Questions
Problem 37QAP: Given three acid-base indicators—methyl orange (end point at pH 4), bromthymol blue (end point at...
Related questions
Question
Solve all parts otherwise I will downvote
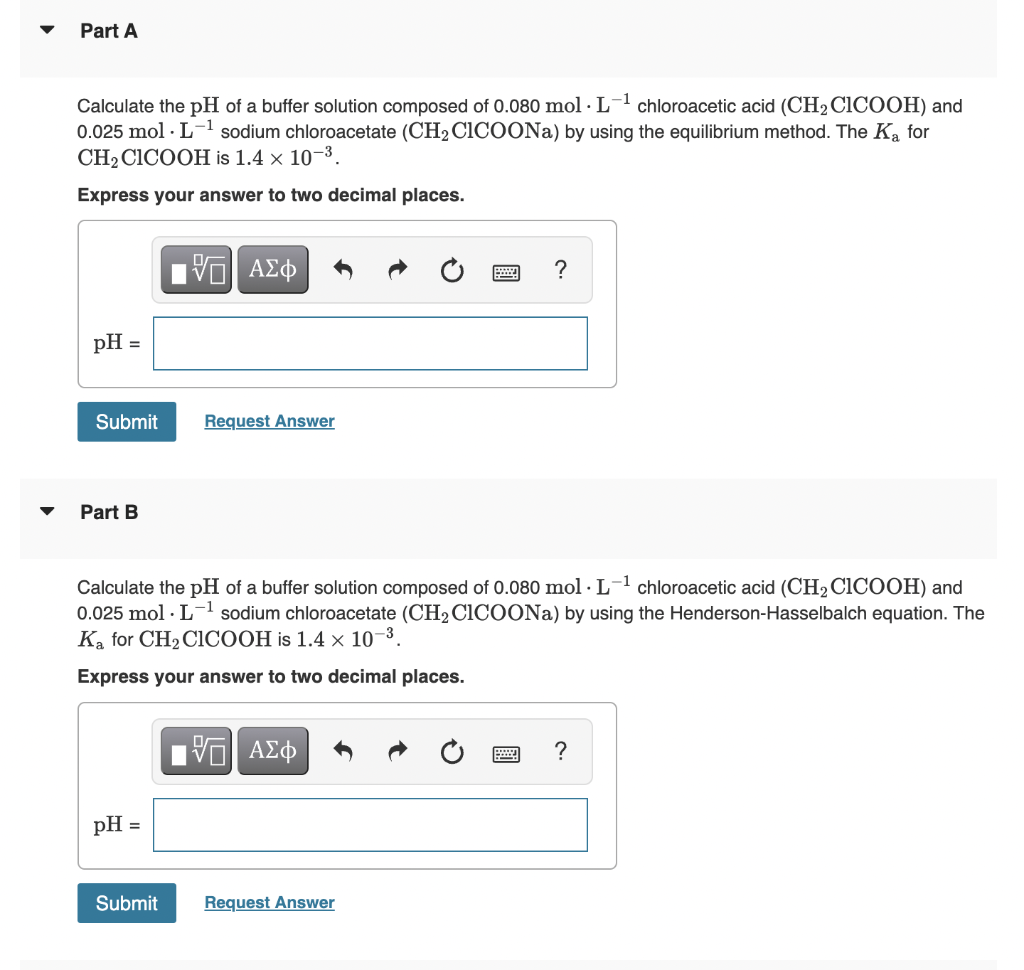
Transcribed Image Text:Part A
Calculate the pH of a buffer solution composed of 0.080 mol · L1 chloroacetic acid (CH2 CICOOH) and
0.025 mol · L-1 sodium chloroacetate (CH2CICOONA) by using the equilibrium method. The Ka for
CH2 CICOOH is 1.4 × 10¬3.
Express your answer to two decimal places.
ΑΣφ
?
pH =
Submit
Request Answer
Part B
Calculate the pH of a buffer solution composed of 0.080 mol · L-1 chloroacetic acid (CH2 CICOOH) and
0.025 mol · L-1 sodium chloroacetate (CH2 CICOONA) by using the Henderson-Hasselbalch equation. The
Ka for CH2 CICOOH is 1.4 × 10-3.
Express your answer to two decimal places.
ΑΣφ
?
pH =
Submit
Request Answer
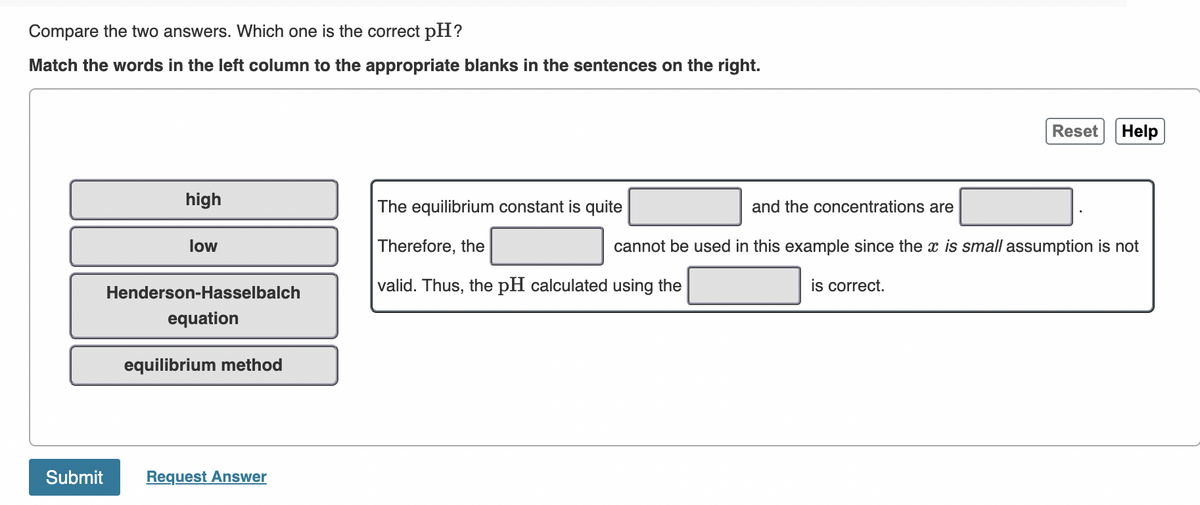
Transcribed Image Text:Compare the two answers. Which one is the correct pH?
Match the words in the left column to the appropriate blanks in the sentences on the right.
Reset
Help
high
The equilibrium constant is quite
and the concentrations are
low
Therefore, the
cannot be used in this example since the x is small assumption is not
valid. Thus, the pH calculated using the
is correct.
Henderson-Hasselbalch
equation
equilibrium method
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax