Consider the following reaction where K. = 1.20x10-2 at 500 K: PCI5 (g) PCI3 (g) + Cl2 (g) A reaction mixture was found to contain 0.112 moles of PCI5 (g), 2.42x10 2 moles of PCI3 (g), and 4.24x102 moles of Cl2 (g), in a 1.00 liter containe
Consider the following reaction where K. = 1.20x10-2 at 500 K: PCI5 (g) PCI3 (g) + Cl2 (g) A reaction mixture was found to contain 0.112 moles of PCI5 (g), 2.42x10 2 moles of PCI3 (g), and 4.24x102 moles of Cl2 (g), in a 1.00 liter containe
ChapterU6: Showtime: Reversible Reactions And Chemical Equilibrium
Section: Chapter Questions
Problem 20STP
Related questions
Question
justify whether the statements are true or not

Transcribed Image Text:In order to reach equilibrium Ke must increase.
Qc is greater than Kc.
The reaction is at equilibrium. No further reaction will occur.
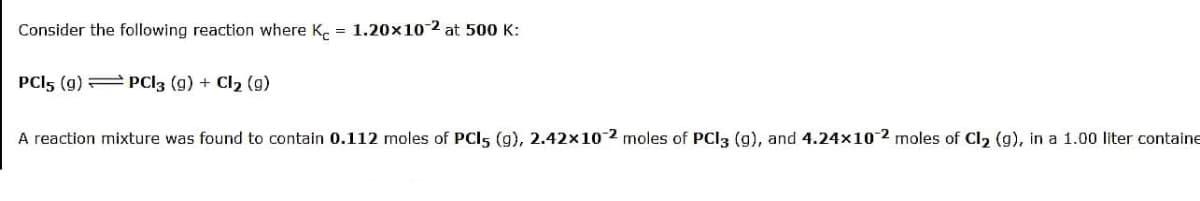
Transcribed Image Text:Consider the following reaction where K. = 1.20x10-2 at 500 K:
PCI5 (g) = PCI3 (g) + Cl2 (g)
A reaction mixture was found to contain 0.112 moles of PCI5 (g), 2.42x10-2 moles of PCI3 (g), and 4.24×102 moles of Cl2 (g), in a 1.00 liter containe
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning