Part A Consider the reaction: Fe-(ag) + SCN- (aq) = FESCN2+ (ag) A solution is made containing initial [Fe= 1.4x10-3 Mand initial ISCN-=8.5x104 M. At equilibrium, [FESCN?+1= 1.6x10-4 M. Calculate the value of the equilibrium constant. Hint: Use the chemical reaction stoichiometry to calculate the equilibrium concentrations of Fe and SCN Express your answer using two significant figures. ? Keg = Submit Request Answer Provide Feedback <40) Problem Set #11 (Ch 11) Exercise 11.69 - Enhanced - with Feedback I Review I Constants I Per Part A MISSED THIS? Watch KCV 11.4, IWE 11.8; Read Section 11.8. You can click on the Review link to access the section in your e Text. What is the molar mass of the gas? An experiment shows that a 254 mL gas sample has a mass of 0.428 g at a pressure of 740 mm Hg and a temperature of 26 °C. nν ΑΣφ ? Molar Mass= g/mol Submit Request Answer Provide Feedback itemView?assignmentProblemID=184058962 <40) Problem Set #11 (Ch 11) Exercise 11.73 I Roviow I Constants I P MISSED THIS? Watch KCV 119; Read Section 11.9. You can click on the Review link to access the section in your e Text. Part A A A gas mixture contains each of the following gases at the indicated partial pressure. N2 219 torr O2 109 torr He 249 torr What is the total pressure of the mixture? Express your answer in torr to three significant figures. ?
Part A Consider the reaction: Fe-(ag) + SCN- (aq) = FESCN2+ (ag) A solution is made containing initial [Fe= 1.4x10-3 Mand initial ISCN-=8.5x104 M. At equilibrium, [FESCN?+1= 1.6x10-4 M. Calculate the value of the equilibrium constant. Hint: Use the chemical reaction stoichiometry to calculate the equilibrium concentrations of Fe and SCN Express your answer using two significant figures. ? Keg = Submit Request Answer Provide Feedback <40) Problem Set #11 (Ch 11) Exercise 11.69 - Enhanced - with Feedback I Review I Constants I Per Part A MISSED THIS? Watch KCV 11.4, IWE 11.8; Read Section 11.8. You can click on the Review link to access the section in your e Text. What is the molar mass of the gas? An experiment shows that a 254 mL gas sample has a mass of 0.428 g at a pressure of 740 mm Hg and a temperature of 26 °C. nν ΑΣφ ? Molar Mass= g/mol Submit Request Answer Provide Feedback itemView?assignmentProblemID=184058962 <40) Problem Set #11 (Ch 11) Exercise 11.73 I Roviow I Constants I P MISSED THIS? Watch KCV 119; Read Section 11.9. You can click on the Review link to access the section in your e Text. Part A A A gas mixture contains each of the following gases at the indicated partial pressure. N2 219 torr O2 109 torr He 249 torr What is the total pressure of the mixture? Express your answer in torr to three significant figures. ?
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section: Chapter Questions
Problem 76PS
Related questions
Question
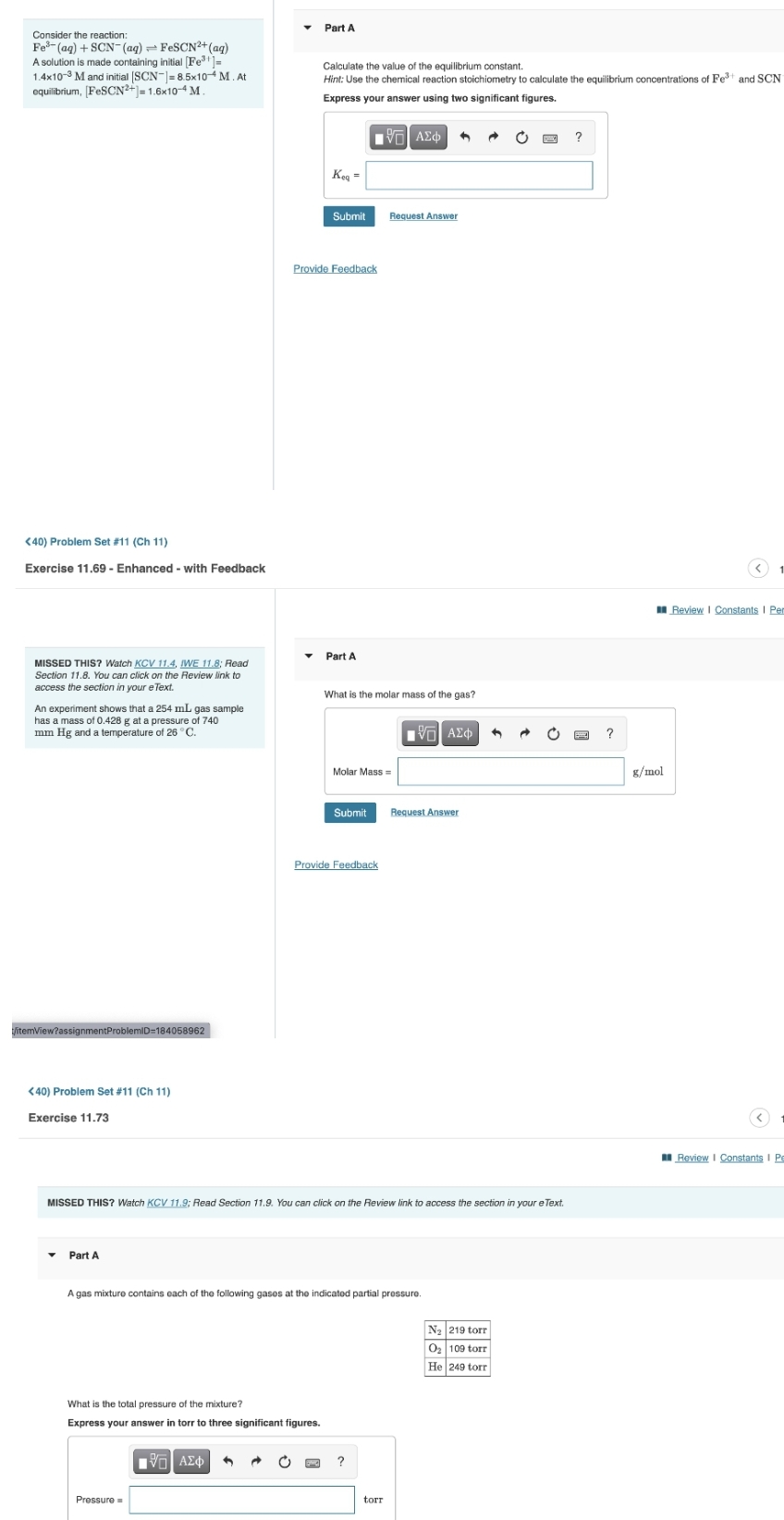
Transcribed Image Text:Part A
Consider the reaction:
Fe- (ag) + SCN- (aq) = FESCN2+(aq)
A solution is made containing initial [Fe=
1.4x10-3 M and initial ISCN-|=8.5x104 M. At
equilibrium, [FESCN?+- 1.6x10-4 M .
Calculate the value of the equilibrium constant.
Hint: Use the chemical reaction stoichiometry to calculate the equilibrium concentrations of Fet and SCN
Express your answer using two significant figures.
?
Keg =
Submit
Request Answer
Provide Feedback
<40) Problem Set #11 (Ch 11)
Exercise 11.69 - Enhanced - with Feedback
I Review I Constants I Per
Part A
MISSED THIS? Watch KCV 11.4, WE 11.8; Read
Section 11.8. You can click on the Review link to
access the section in your e Text.
What is the molar mass of the gas?
An experiment shows that a 254 mL gas sample
has a mass of 0.428 g at a pressure of 740
mm Hg and a temperature of 26 °C.
nνα ΑΣφ
?
Molar Mass =
g/mol
Submit
Request Answer
Provide Feedback
itemView?assignmentProblemiD=184058962
<40) Problem Set #11 (Ch 11)
Exercise 11.73
I Roviow I Constants I Pe
MISSED THIS? Watch KCV 11.9; Read Section 11.9. You can click on the Review link to access the section in your e Text.
Part A
A
A gas mixture contains each of the following gases at the indicated partial pressure.
N2 219 torr
O2 109 torr
He 249 torr
What is the total pressure of the mixture?
Express your answer in torr to three significant figures.
Prossure
torr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning