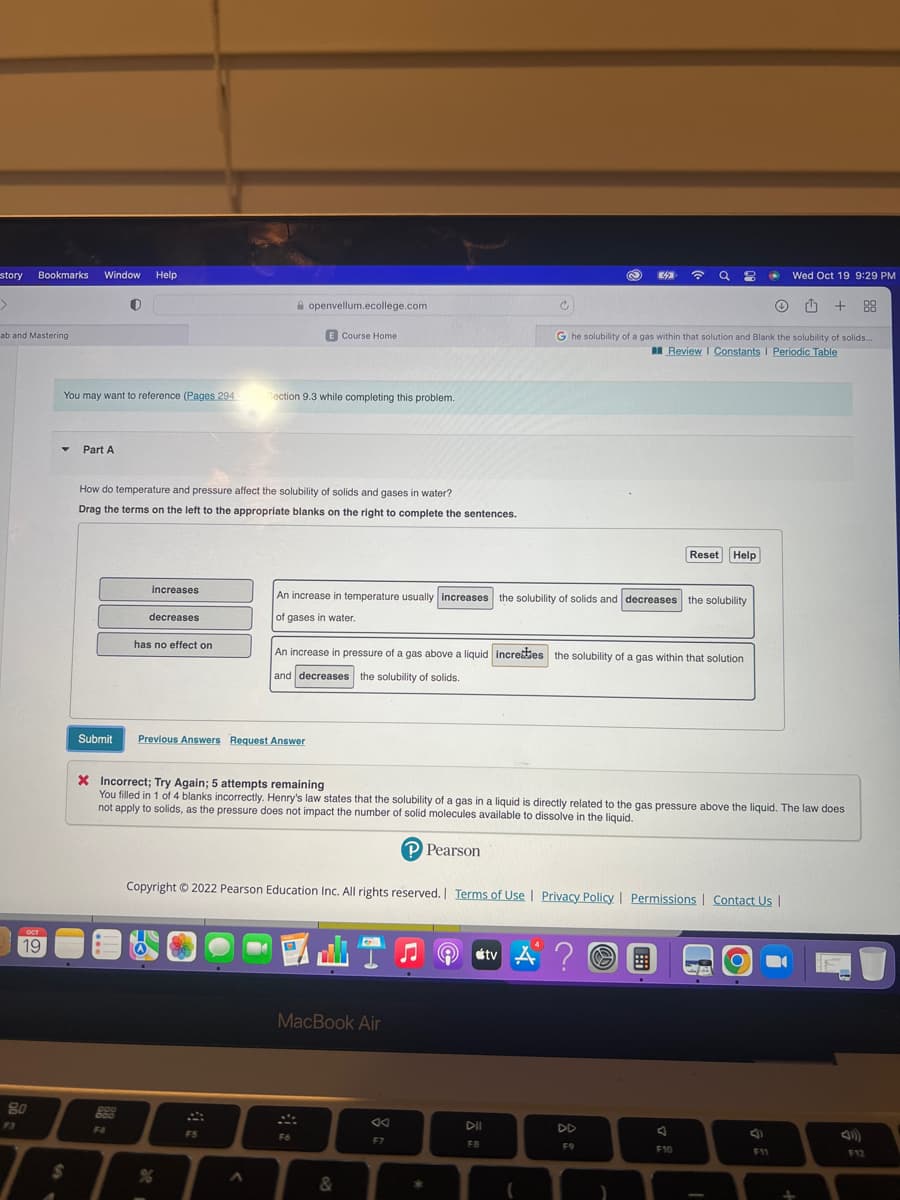
Part A How do temperature and pressure affect the solubility of solids and gases in water? Drag the terms on the left to the appropriate blanks on the right to complete the sentences. increases decreases has no effect on Reset Help An increase in temperature usually increases the solubility of solids and decreases the solubility of gases in water. An increase in pressure of a gas above a liquid incredes the solubility of a gas within that solution and decreases the solubility of solids.
Part A How do temperature and pressure affect the solubility of solids and gases in water? Drag the terms on the left to the appropriate blanks on the right to complete the sentences. increases decreases has no effect on Reset Help An increase in temperature usually increases the solubility of solids and decreases the solubility of gases in water. An increase in pressure of a gas above a liquid incredes the solubility of a gas within that solution and decreases the solubility of solids.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter12: Solutions
Section: Chapter Questions
Problem 12.115QP: Two samples of sodium chloride solutions are brought to a boil on a stove. One of the solutions...
Related questions
Question
Transcribed Image Text:story Bookmarks Window Help
>
ab and Mastering
OCT
19
80
You may want to reference (Pages 294-
A
▼
$
Part A
Submit
increases
How do temperature and pressure affect the solubility of solids and gases in water?
Drag the terms on the left to the appropriate blanks on the right to complete the sentences.
decreases
has no effect on
Section 9.3 while completing this problem.
Previous Answers Request Answer
%
openvellum.ecollege.com
FS
E Course Home
An increase temperature usually increases the solubility of solids and decreases the solubility
of gases in water.
S
An increase in pressure of a gas above a liquid increes the solubility of a gas within that solution
and decreases the solubility of solids.
F6
X Incorrect; Try Again; 5 attempts remaining
You filled in 1 of 4 blanks incorrectly. Henry's law states that the solubility of a gas in a liquid is directly related to the gas pressure above the liquid. The law does
not apply to solids, as the pressure does not impact the number of solid molecules available to dissolve in the liquid.
P Pearson
Copyright © 2022 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions | Contact Us |
MacBook Air
&
F7
*
@ 442 ☎ Q
DII
F8
+88
G he solubility of a gas within that solution and Blank the solubility of solids...
Review | Constants I Periodic Table
tv A ?
(
Reset Help
DD
F9
A
F10
S
7
Wed Oct 19 9:29 PM
F11
+
IF
F12
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
