• Part A ithium bromide and lead() acetate Express your answer as a chemical equation. Identify all of the phases in your answer. Enter NOREACTION if no reaction occurs. > View Available Hintis) ΑΣΦ O I8 DA chemical reaction does not occur for this question. Submit Previous Answers X Incorrect; Try Again; 3 attempts remaining Consult the solubility rules to determine the solublity of the products of this reaction. If a product is insoluble, use the solid physical state to describe it. • Part B potassium sultate and calcium chloride Express your answer as a chemical equation. Identify all of the phases in your answer. Enter NOREACTION it no reaction occurs. > View Available Hint(s) AXO + • O IE ? JA chemical reaction does not occur for this question. Submit Previous Answers X Incorrect; Try Again; 5 attempts remaining Consult the solubility rules to determine the solubility of the products of this reaction. If a product is insoluble, use the solid physical state to describe it. • Part C ithium chloride and calcium sulfide Express your answer as a chemical equation. Identify all of the phases in your answer. Enter NOREACTION it no reaction occurs. > View Available Hintis) NOREACTION Submit Previous Answers v Correct The possible products for this reaction are Li-S and CaCla, which are both soluble. Therefore, the reaction does not proceed, Part D iron(l) nitrate and sodium phosphate Express your answer as a chemical equation. Identify all of the phases in your answer. Enter NOREACTION if no reaction occurs. > View Available Hint(s) ΑΣΦ O IE DA chemical reaction does not occur for this question. Submit Provide Feedback
• Part A ithium bromide and lead() acetate Express your answer as a chemical equation. Identify all of the phases in your answer. Enter NOREACTION if no reaction occurs. > View Available Hintis) ΑΣΦ O I8 DA chemical reaction does not occur for this question. Submit Previous Answers X Incorrect; Try Again; 3 attempts remaining Consult the solubility rules to determine the solublity of the products of this reaction. If a product is insoluble, use the solid physical state to describe it. • Part B potassium sultate and calcium chloride Express your answer as a chemical equation. Identify all of the phases in your answer. Enter NOREACTION it no reaction occurs. > View Available Hint(s) AXO + • O IE ? JA chemical reaction does not occur for this question. Submit Previous Answers X Incorrect; Try Again; 5 attempts remaining Consult the solubility rules to determine the solubility of the products of this reaction. If a product is insoluble, use the solid physical state to describe it. • Part C ithium chloride and calcium sulfide Express your answer as a chemical equation. Identify all of the phases in your answer. Enter NOREACTION it no reaction occurs. > View Available Hintis) NOREACTION Submit Previous Answers v Correct The possible products for this reaction are Li-S and CaCla, which are both soluble. Therefore, the reaction does not proceed, Part D iron(l) nitrate and sodium phosphate Express your answer as a chemical equation. Identify all of the phases in your answer. Enter NOREACTION if no reaction occurs. > View Available Hint(s) ΑΣΦ O IE DA chemical reaction does not occur for this question. Submit Provide Feedback
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 134IP: On Easter Sunday, April 3, 1983, nitric acid spilled from a tank car near downtown Denver, Colorado....
Related questions
Question
Not C
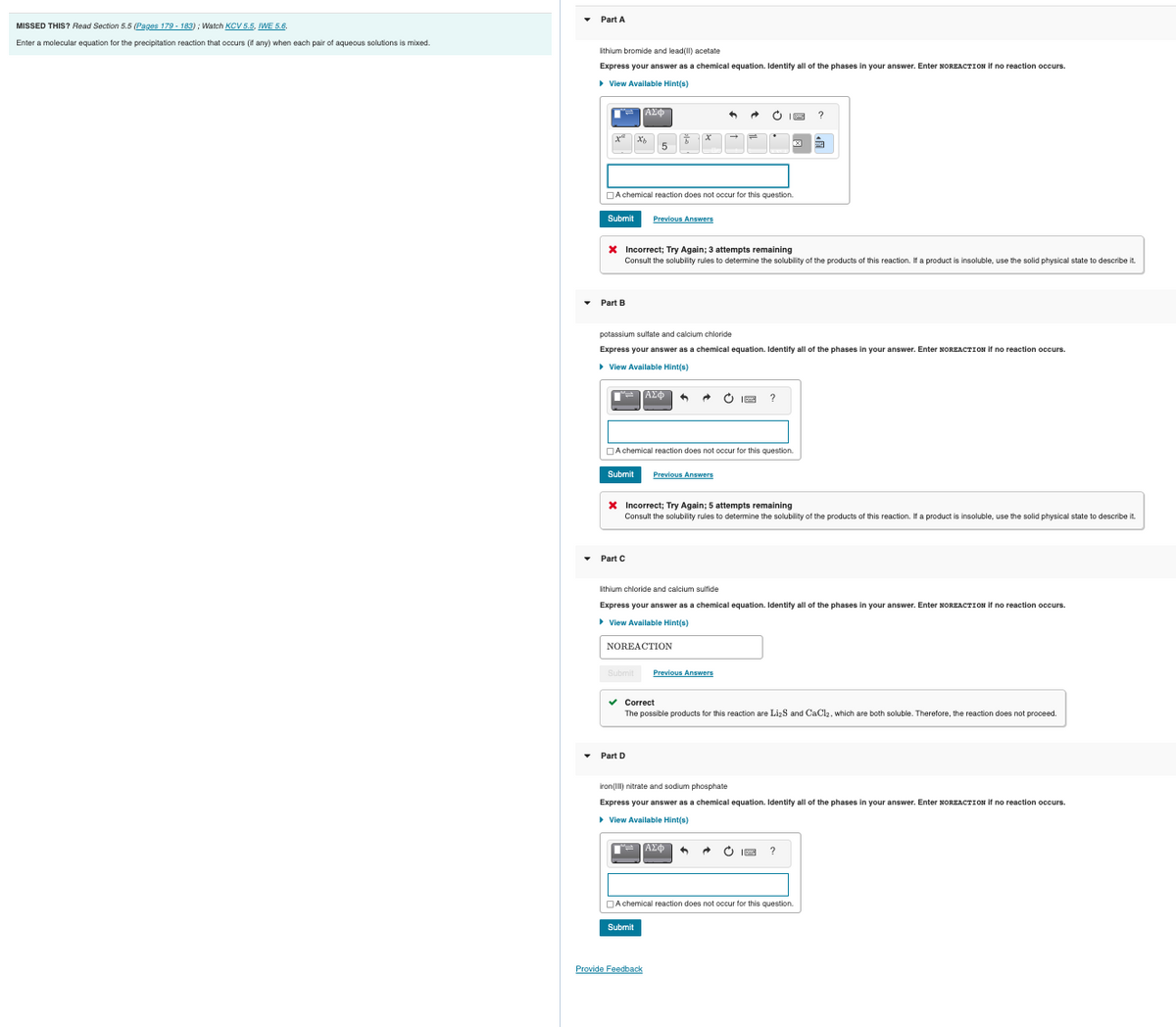
Transcribed Image Text:Part A
MISSED THIS? Read Section 5.5 (Pages 179 - 183) ; Watch KCV 5.5, IWE 5.6.
Enter a molecular equation for the precipitation reaction that occurs (it any) when each pair of aqueous solutions is mixed.
Ithium bromide and lead(II) acetate
Express your answer as a chemical equation, Identify all of the phases in vour answer. Enter NOREACTION it no reaction occurs.
> View Available Hint(s)
ΑΣΦ
?
5
DA chemical reaction does not occur for this question.
Submit
Previous Answers
X Incorrect; Try Again; 3 attempts remaining
Consult the solubility rules to detemine the solubility of the products of this reaction. If a product is insoluble, use the solid physical state to describe it.
• Part B
potassium sulfate and calcium chloride
Express your answer as a chemical equation. Identify all of the phases in your answer. Enter NOREACTION if no reaction occurs.
> View Available Hint(s)
ΑΣΦ
DA chemical reaction does not occur for this question.
Submit
Previous Answers
X Incorrect; Try Again; 5 attempts remaining
Consult the solubility rules to determine the solubility of the products of this reaction. If a product is insoluble, use the solid physical state to describe it.
Part C
ithium chloride and calcium sulfide
Express your answer as a chemical equation. Identify all of the phases in your answer. Enter NOREACTION if no reaction occurs.
> View Available Hint(s)
NOREACTION
Submit
Previous Answers
v Correct
The possible products for this reaction are LizS and CaCl2, which are both soluble. Therefore, the reaction does not proceed.
• Part D
iron(Il) nitrate and sodium phosphate
Express your answer as a chemical equation. Identify all of the phases in your answer. Enter NOREACTION if no reaction occurs.
• View Available Hint(s)
O IE
DA chemical reaction does not occur for this question.
Submit
Provide Feedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning