Part A Nitrogen dioxide obtained as a cylinder gas is always a mixture of NO2(9) and N3O4(g). 4.90 -g sample obtained from such a cylinder is sealed in a 0.400 -L flask at 298 K N,04 (9) 2 NO, Kc 4.61 x 10 3 What is the mole fraction of NO2(9) in this mixture? mol fraction NO, = 0.667 Submit Previous Answers Request Answer x Incorrect; Try Again; 2 attempts remaining
Part A Nitrogen dioxide obtained as a cylinder gas is always a mixture of NO2(9) and N3O4(g). 4.90 -g sample obtained from such a cylinder is sealed in a 0.400 -L flask at 298 K N,04 (9) 2 NO, Kc 4.61 x 10 3 What is the mole fraction of NO2(9) in this mixture? mol fraction NO, = 0.667 Submit Previous Answers Request Answer x Incorrect; Try Again; 2 attempts remaining
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 76QAP: The Lamborghini Aventador engine has a 12-cylinder engine in which each cylinder has a volume of 542...
Related questions
Question
1
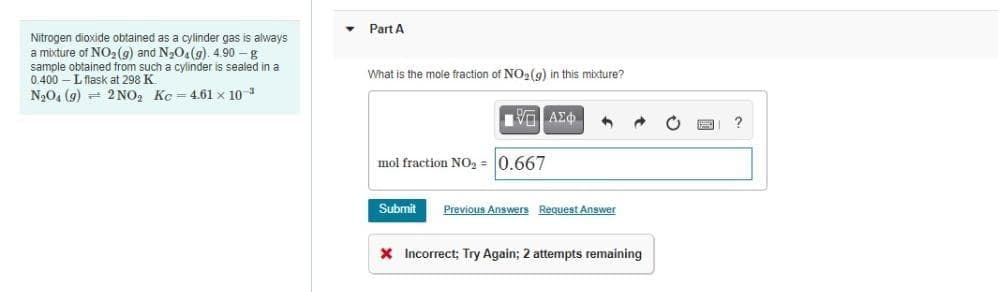
Transcribed Image Text:Part A
Nitrogen dioxide obtained as a cylinder gas is always
a mixture of NO2(g) and N3O4(g). 4.90 – g
sample obtained from such a cylinder is sealed in a
0.400 – Lflask at 298 K.
N20, (9) - 2 NO, Kc = 4.61 x 10
What is the mole fraction of NO2(9) in this mixture?
mol fraction NO2 = 0.667
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 2 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning