Part A Select the correct electron configurations from the list below. You can refer to the periodic table for atomic numbers. Check all that apply. ► View Available Hint(s) The electron configuration of C is [He]2s²2p². The electron configuration of Aut is [Xe]4f¹45dº6s¹. The electron configuration of Cs is [Xe]6sº5d¹. The electron configuration of $² is [Ne]3s²3p6. The electron configuration of Fe is [Ar]4s²3dº. Submit U
Part A Select the correct electron configurations from the list below. You can refer to the periodic table for atomic numbers. Check all that apply. ► View Available Hint(s) The electron configuration of C is [He]2s²2p². The electron configuration of Aut is [Xe]4f¹45dº6s¹. The electron configuration of Cs is [Xe]6sº5d¹. The electron configuration of $² is [Ne]3s²3p6. The electron configuration of Fe is [Ar]4s²3dº. Submit U
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 21P: Identify the atom or ion corresponding to each of the following descriptions: (a) an atom with...
Related questions
Question
Pls help ASAP. Pls do all asked questions I BEG
![Part A
Select the correct electron configurations from the list below. You can refer to the periodic table for atomic numbers.
Check all that apply.
► View Available Hint(s)
The electron configuration of C is [He]2s²2p².
The electron configuration of Aut is [Xe]4f¹45d³6s¹.
The electron configuration of Cs is [Xe]6sº5d¹.
The electron configuration of S²- is [Ne]3s²3p6.
The electron configuration of Fe is [Ar] 4s²3dº.
Submit](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fd400780e-3958-4546-be7a-8b30973c1ded%2F0597f93f-01da-408b-952e-de5dfb7fa3bd%2F044xv48_processed.png&w=3840&q=75)
Transcribed Image Text:Part A
Select the correct electron configurations from the list below. You can refer to the periodic table for atomic numbers.
Check all that apply.
► View Available Hint(s)
The electron configuration of C is [He]2s²2p².
The electron configuration of Aut is [Xe]4f¹45d³6s¹.
The electron configuration of Cs is [Xe]6sº5d¹.
The electron configuration of S²- is [Ne]3s²3p6.
The electron configuration of Fe is [Ar] 4s²3dº.
Submit
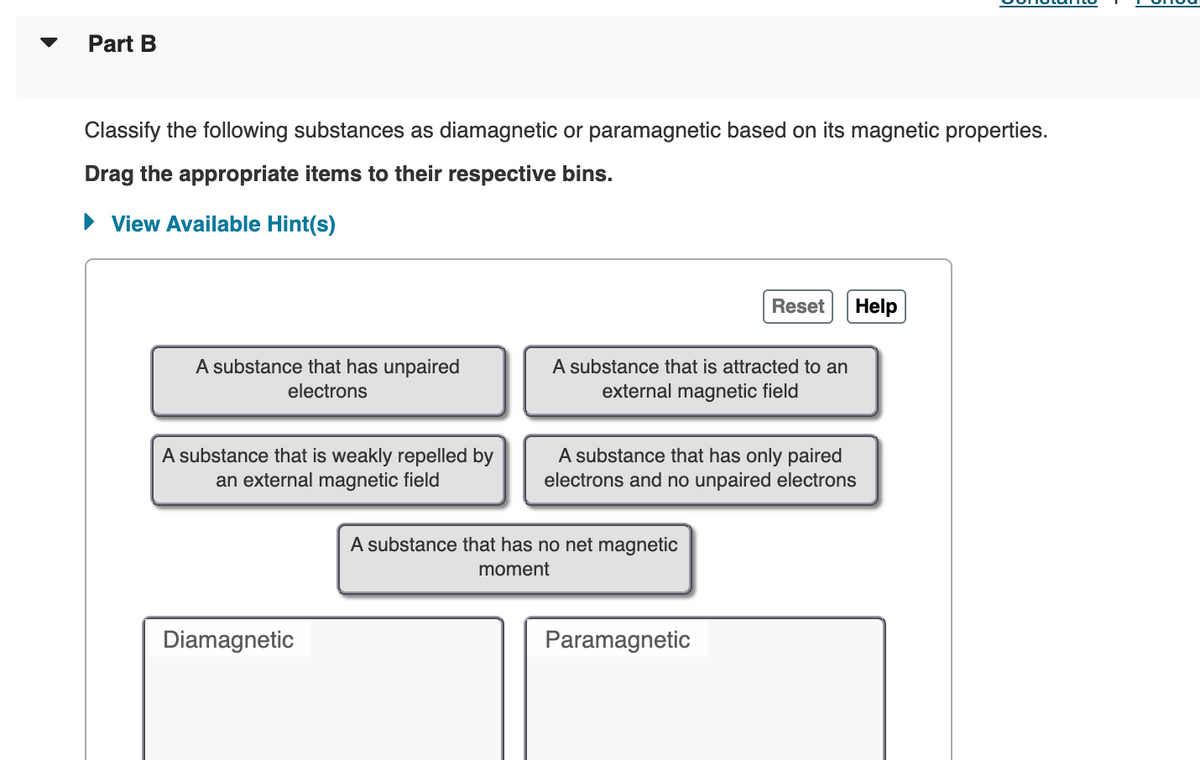
Transcribed Image Text:Part B
Classify the following substances as diamagnetic or paramagnetic based on its magnetic properties.
Drag the appropriate items to their respective bins.
View Available Hint(s)
Reset Help
A substance that has unpaired
electrons
A substance that is attracted to an
external magnetic field
A substance that is weakly repelled by
an external magnetic field
A substance that has only paired
electrons and no unpaired electrons
Diamagnetic
A substance that has no net magnetic
moment
Paramagnetic
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning