Part A The electron in a hydrogen atom spends most of its time 0.53 x 1010 m from the nucleus, whose radius is about 0.88 x 10 5 °m. If each dimension of this atom was increased by the same factor and the radius of the nucleus was increased to the size of a tennis ball, how far from the nucleus would the electron be? Assume that the radius of a tennis ball is 3.0 cm. Express your answer with the appropriate units. Templates Symbols undo reglo reset keyboard shortcuts help Value Units
Part A The electron in a hydrogen atom spends most of its time 0.53 x 1010 m from the nucleus, whose radius is about 0.88 x 10 5 °m. If each dimension of this atom was increased by the same factor and the radius of the nucleus was increased to the size of a tennis ball, how far from the nucleus would the electron be? Assume that the radius of a tennis ball is 3.0 cm. Express your answer with the appropriate units. Templates Symbols undo reglo reset keyboard shortcuts help Value Units
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter29: Atomic Physics
Section: Chapter Questions
Problem 48P
Related questions
Question
100%
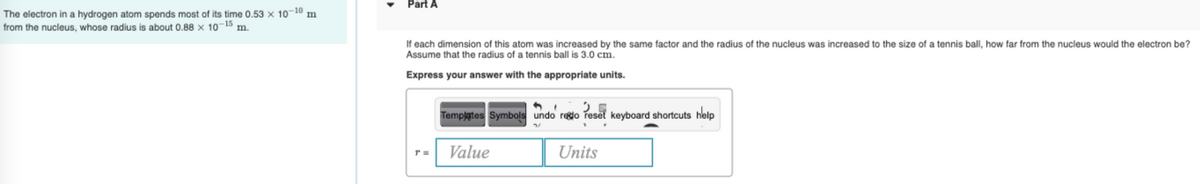
Transcribed Image Text:Part A
The electron in a hydrogen atom spends most of its time 0.53 x 10-10 m
from the nucleus, whose radius is about 0.88 x 10-15 m.
If each dimension of this atom was increased by the same factor and the radius of the nucleus was increased to the size of a tennis ball, how far from the nucleus would the electron be?
Assume that the radius of a tennis ball is 3.0 cm.
Express your answer with the appropriate units.
Templates Symbols undo redo reset keyboard shortcuts help
Value
Units

Transcribed Image Text:Why does the term "electron orbit" have no meaning in quantum mechanics?
The electron is a cloud smeared in a three-dimensional space.
The electron has an equal probability of being found in every point of space.
O The electron is considered to be at rest.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning