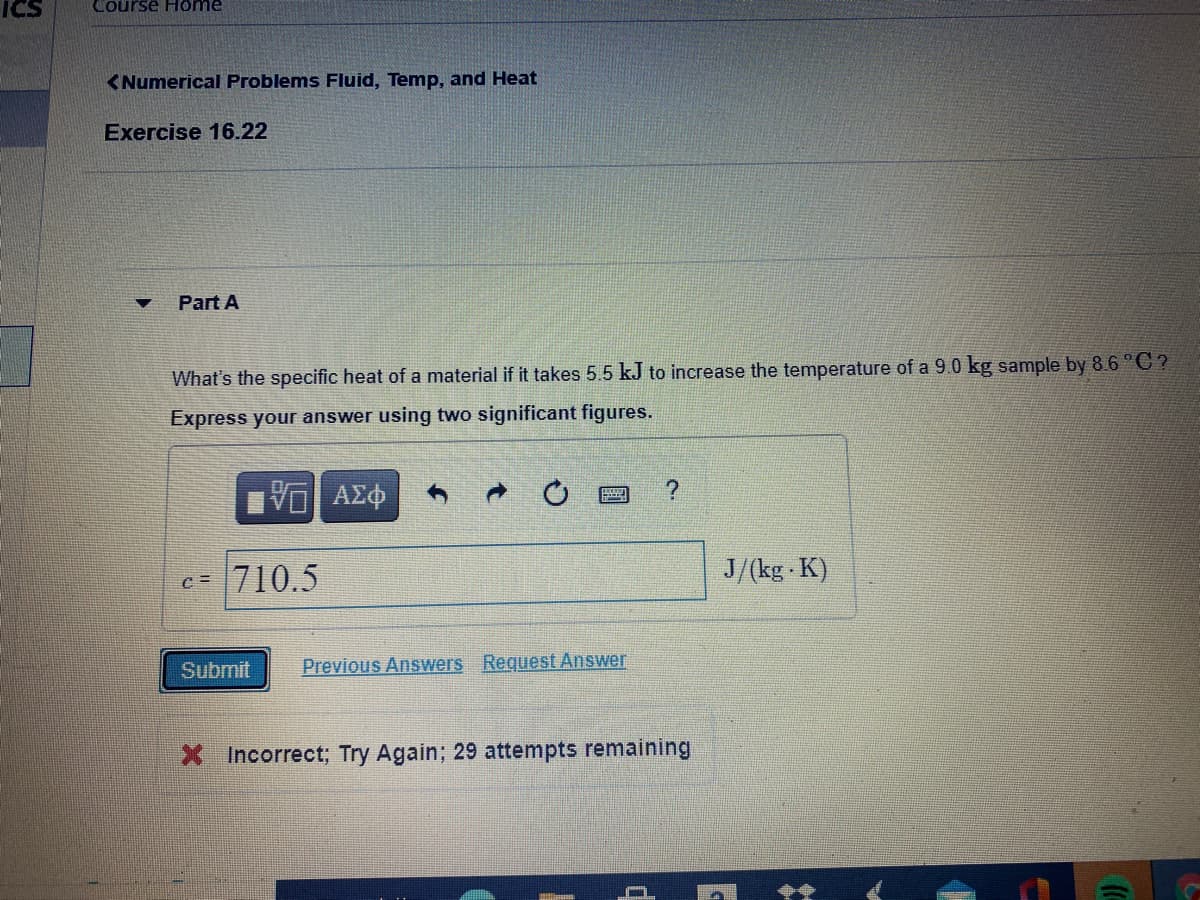
Part A What's the specific heat of a material if it takes 5.5 kJ to increase the temperature of a 9.0 kg sample by 8.6 °C? Express your answer using two significant figures.
Part A What's the specific heat of a material if it takes 5.5 kJ to increase the temperature of a 9.0 kg sample by 8.6 °C? Express your answer using two significant figures.
University Physics Volume 1
18th Edition
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:William Moebs, Samuel J. Ling, Jeff Sanny
Chapter1: Units And Measurement
Section: Chapter Questions
Problem 56P: Estimates and Fermi Calculations Assuming the human body is made primarily of water, estimate the...
Related questions
Question
Can someone help me with this question
Transcribed Image Text:ICS
Course H ome
<Numerical Problems Fluid, Temp, and Heat
Exercise 16.22
Part A
What's the specific heat of a material if it takes 5.5 kJ to increase the temperature of a 9.0 kg sample by 8.6 °C ?
Express your answer using two significant figures.
c=
710.5
J/(kg K)
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 29 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University

University Physics Volume 1
Physics
ISBN:
9781938168277
Author:
William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:
OpenStax - Rice University