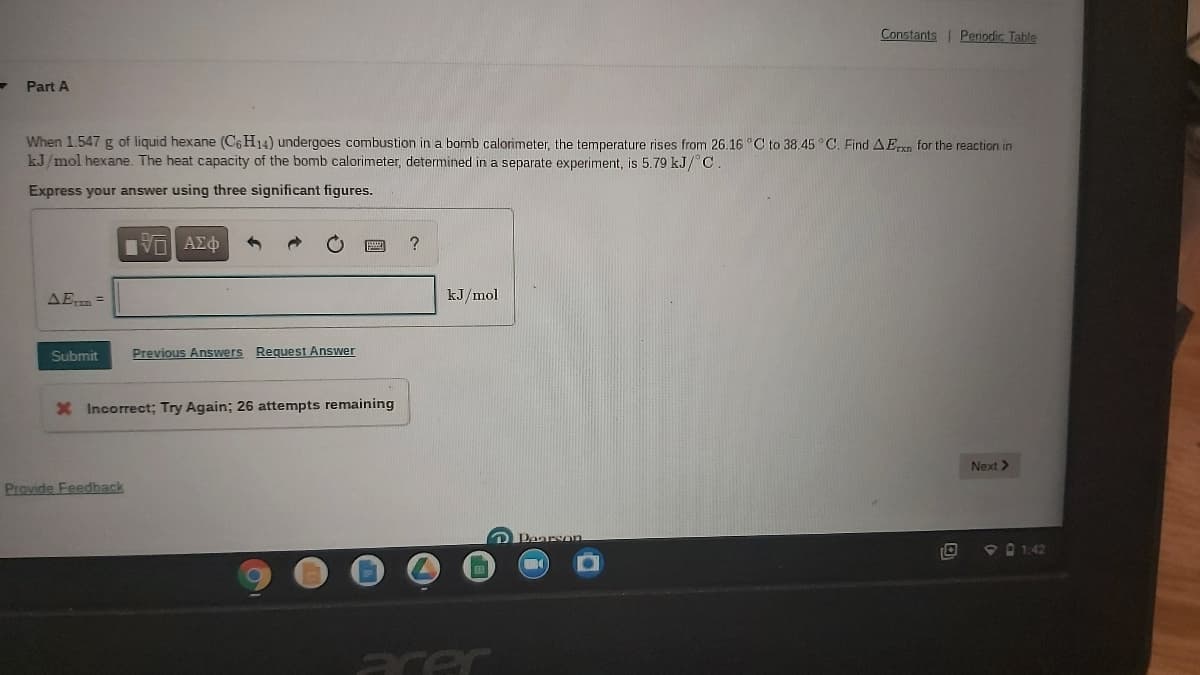
Part A When 1.547 g of liquid hexane (C6 H14) undergoes combustion in a bomb calorimeter, the temperature rises from 26.16 C to 38.45 °C. Find AErxn for the reaction in kJ/mol hexane. The heat capacity of the bomb calorimeter, determined in a separate experiment, is 5.79 kJ/ C. Express your answer using three significant figures.
Part A When 1.547 g of liquid hexane (C6 H14) undergoes combustion in a bomb calorimeter, the temperature rises from 26.16 C to 38.45 °C. Find AErxn for the reaction in kJ/mol hexane. The heat capacity of the bomb calorimeter, determined in a separate experiment, is 5.79 kJ/ C. Express your answer using three significant figures.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section: Chapter Questions
Problem 98IL: A bomb calorimetric experiment was run to determine the enthalpy of combustion of ethanol. The...
Related questions
Question
100%
Transcribed Image Text:Constants Periodic. Table
Part A
When 1.547 g of liquid hexane (C6 H14) undergoes combustion in a bomb calorimeter, the temperature rises from 26.16 °C to 38.45 °C. Find AEpxn for the reaction in
kJ/mol hexane. The heat capacity of the bomb calorimeter, determined in a separate experiment, is 5.79 kJ/ C
Express your answer using three significant figures.
Eνα AΣφ
AE =
kJ/mol
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 26 attempts remaining
Next>
Provide Feedback
Paarsom
O O 1:42
acer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning