Part A Which element is oxidized in this reaction? he 2CuO + C→2Cu + CO2 Enter the chemical symbol of the element. > View Available Hint(s) na is oxidized a in Submit Part B Complete previous part(s) Part C Which element is reduced in this reaction? 2KMNO4 + 3Na2SO3 + H2O 2MnO2 + 3Nag SO4 + 2KOH Enter the chemical symbol of the element. P Pearson
Part A Which element is oxidized in this reaction? he 2CuO + C→2Cu + CO2 Enter the chemical symbol of the element. > View Available Hint(s) na is oxidized a in Submit Part B Complete previous part(s) Part C Which element is reduced in this reaction? 2KMNO4 + 3Na2SO3 + H2O 2MnO2 + 3Nag SO4 + 2KOH Enter the chemical symbol of the element. P Pearson
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 9RQ: What characterizes an electrolytic cell? What is an ampere? When the current applied to an...
Related questions
Question
Transcribed Image Text:Cons
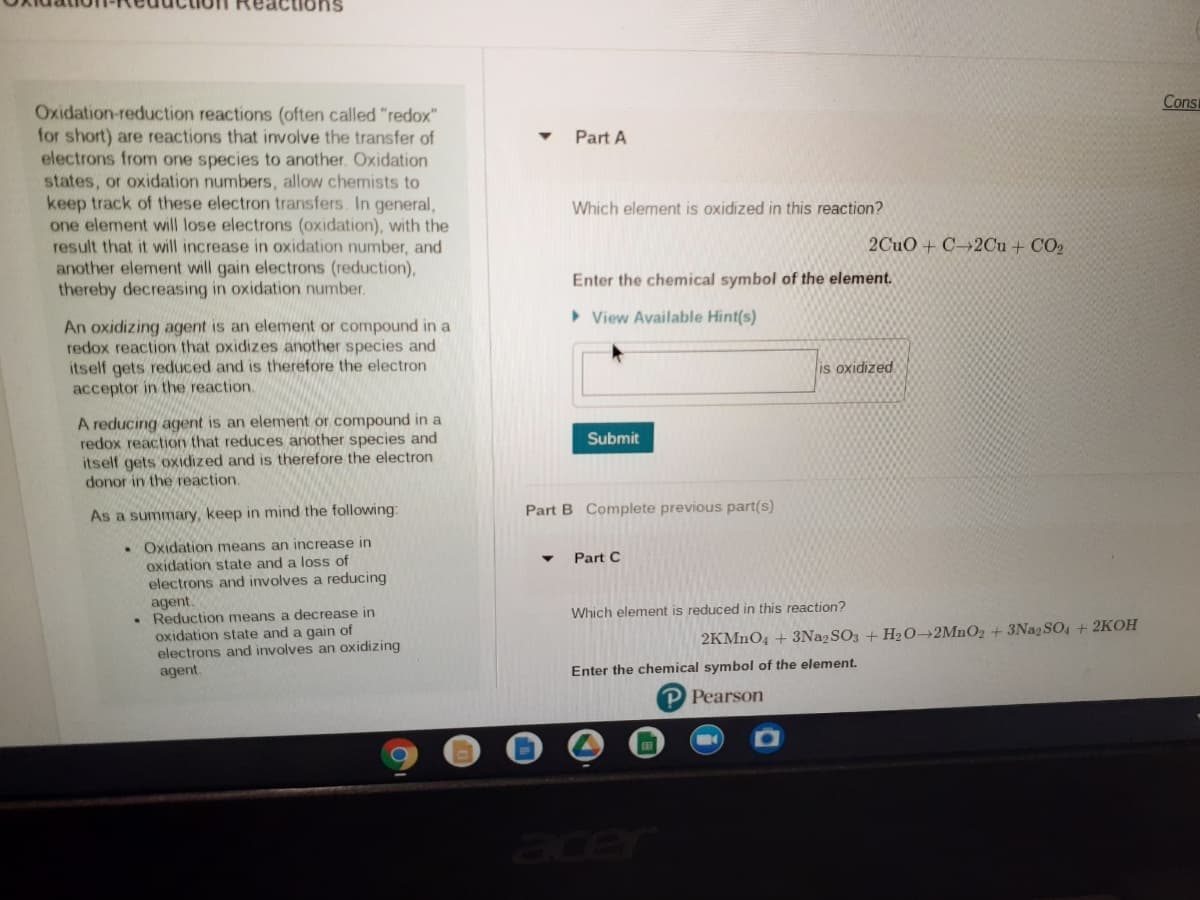
Oxidation-reduction reactions (often called "redox"
for short) are reactions that ivolve the transfer of
electrons from one species to another. Oxidation
states, or oxidation numbers, allow chemists to
keep track of these electron transfers. In general,
one element will lose electrons (oxidation), with the
result that it will increase in oxidation number, and
Part A
Which element is oxidized in this reaction?
2CuO + C→2Cu + CO2
another element will gain electrons (reduction),
thereby decreasing in oxidation number.
Enter the chemical symbol of the element.
> View Available Hint(s)
An oxidizing agent is an element or compound in a
redox reaction that oxidizes another species and
itself gets reduced and is therefore the electron
acceptor in the reaction.
is oxidized
A reducing agent is an element or compound in a
redox reaction that reduces another species and
itself gets oxidized and is therefore the electron
donor in the reaction.
Submit
As a summary, keep in mind the following:
Part B Complete previous part(s)
• Oxidation means an increase in
oxidation state and a loss of
electrons and involves a reducing
agent.
• Reduction means a decrease in
oxidation state and a gain of
electrons and involves an oxidizing
agent.
Part C
Which element is reduced in this reaction?
2KMNO4 + 3Na2SO3 + H2O→2MNO2+3Na2 SO4 + 2KOH
Enter the chemical symbol of the element.
P Pearson
er
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning