Part B Use standard enthalpies of formation to calculate AH for the following reactie CO(g) + H2(g) H2(g)+ CO (g) Express your answer using three significant figures. kJ AH =
Part B Use standard enthalpies of formation to calculate AH for the following reactie CO(g) + H2(g) H2(g)+ CO (g) Express your answer using three significant figures. kJ AH =
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 46E: The reaction SO3(g)+H2O(l)H2SO4(aq) is the last step in the commercial production of sulfuric acid....
Related questions
Question
100%
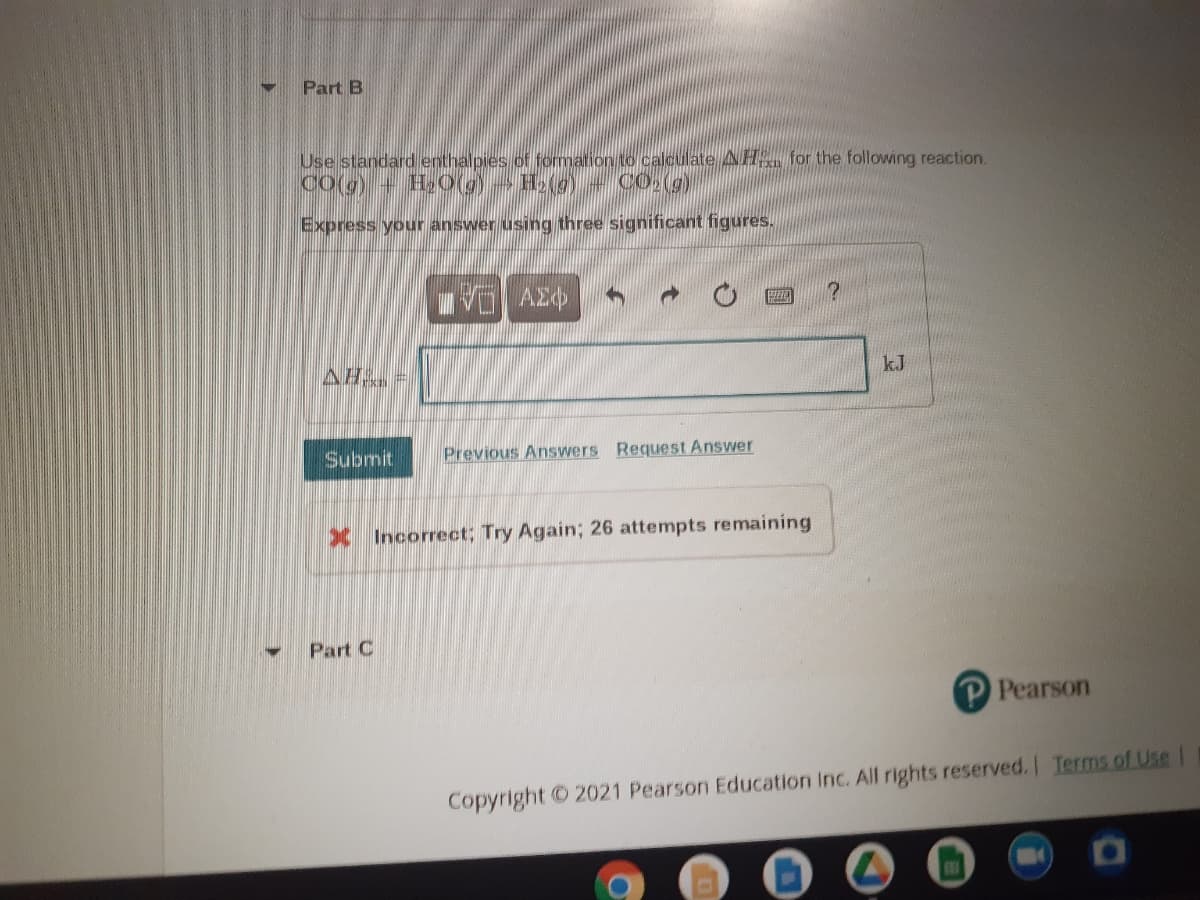
Transcribed Image Text:Part B
Use standard enthalpies of formation to calculate AH for the following reaction.
Co(g)+ H0(g) Holg)
CO2(g)
Express your answer using three significant figures.
kJ
AH =
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 26 attempts remaining
Part C
P Pearson
Copyright 2021 Pearson Education Inc. All rights reserved. Terms of Use
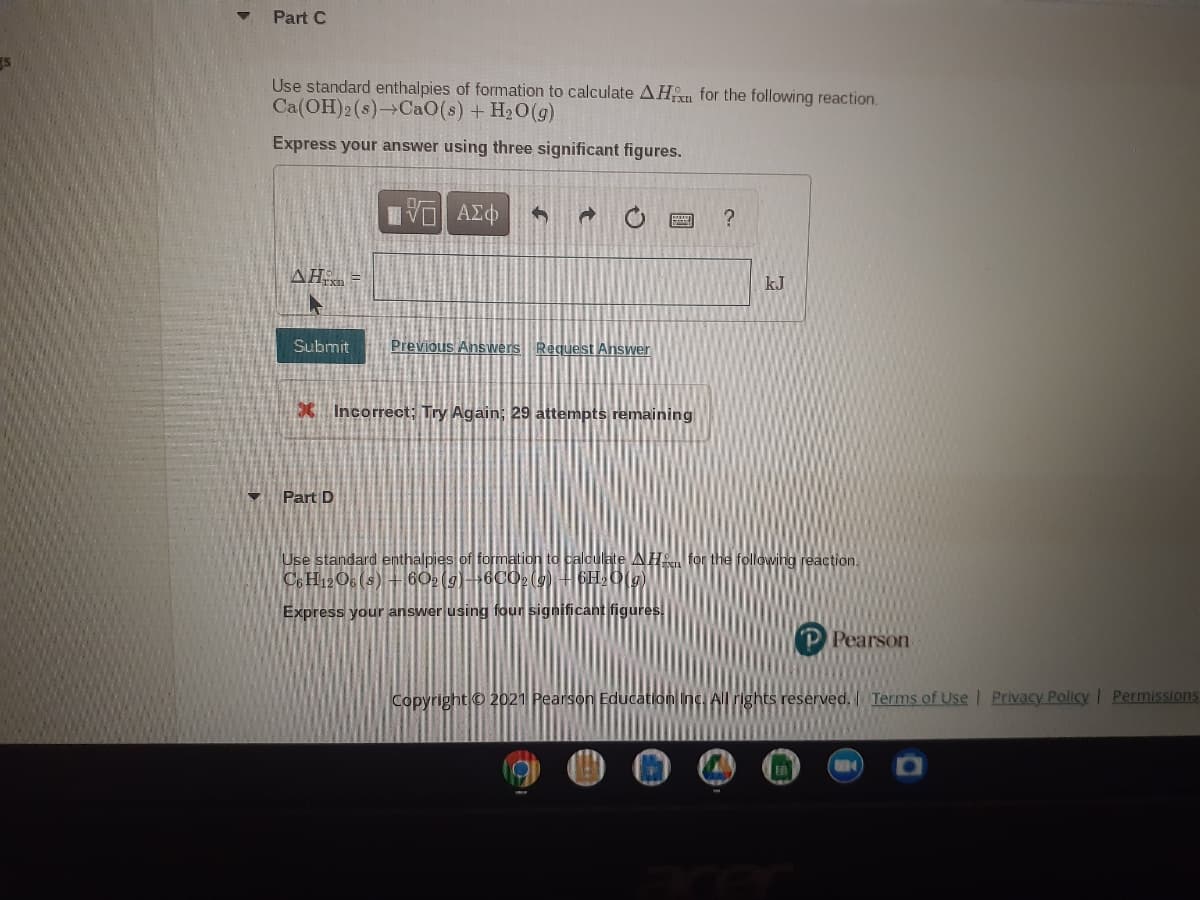
Transcribed Image Text:Part C
Use standard enthalpies of formation to calculate AHn for the following reaction.
Ca(OH)2(s)→CAO(s) + H20(g)
Express your answer using three significant figures.
?
AH =
kJ
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 29 attempts remaining
Part D
Use standard enthalpies of formation to caloulate AH for the following reaction.
Co H12O6 (s) +60(g)6C029) H6H,0(g)
Express your answer using four significant figures.
Pearson
Copyright o 2021 Pearson Education Inc. All rights reserved. Terms of Use / Privacy PolicyI Permissions
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co