✓ Part C Bond length is the distance between the centers of two bonded atoms. On the potential energy curve, the bond length is the internuclear distance between the two atoms when the potential energy of the system reaches its lowest value. Consider that the atomic radius (sometimes called the covalent or bonding atomic radius) of an element is defined as one-half the distance between the bonded atoms in a homonuclear diatomic molecule. Actual bond lengths in molecules are determined experimentally by such methods as X-ray diffraction and microwave spectroscopy. However, these atomic radii values can be used to give an estimate of the upper limit of bond length in other (heteronuclear) molecules. Given that the atomic radii of H and F are 37.0 pm and 72.0 pm, respectively, predict the upper limit of the bond length of the HF molecule. Express your answer to three significant figures and include the appropriate units. ► View Available Hint(s) Bond length upper limit= Submit μA Value Units ?
✓ Part C Bond length is the distance between the centers of two bonded atoms. On the potential energy curve, the bond length is the internuclear distance between the two atoms when the potential energy of the system reaches its lowest value. Consider that the atomic radius (sometimes called the covalent or bonding atomic radius) of an element is defined as one-half the distance between the bonded atoms in a homonuclear diatomic molecule. Actual bond lengths in molecules are determined experimentally by such methods as X-ray diffraction and microwave spectroscopy. However, these atomic radii values can be used to give an estimate of the upper limit of bond length in other (heteronuclear) molecules. Given that the atomic radii of H and F are 37.0 pm and 72.0 pm, respectively, predict the upper limit of the bond length of the HF molecule. Express your answer to three significant figures and include the appropriate units. ► View Available Hint(s) Bond length upper limit= Submit μA Value Units ?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 87AP: At large interatomic separations, an alkali halide molecule MX has a lower energy as two neutral...
Related questions
Question
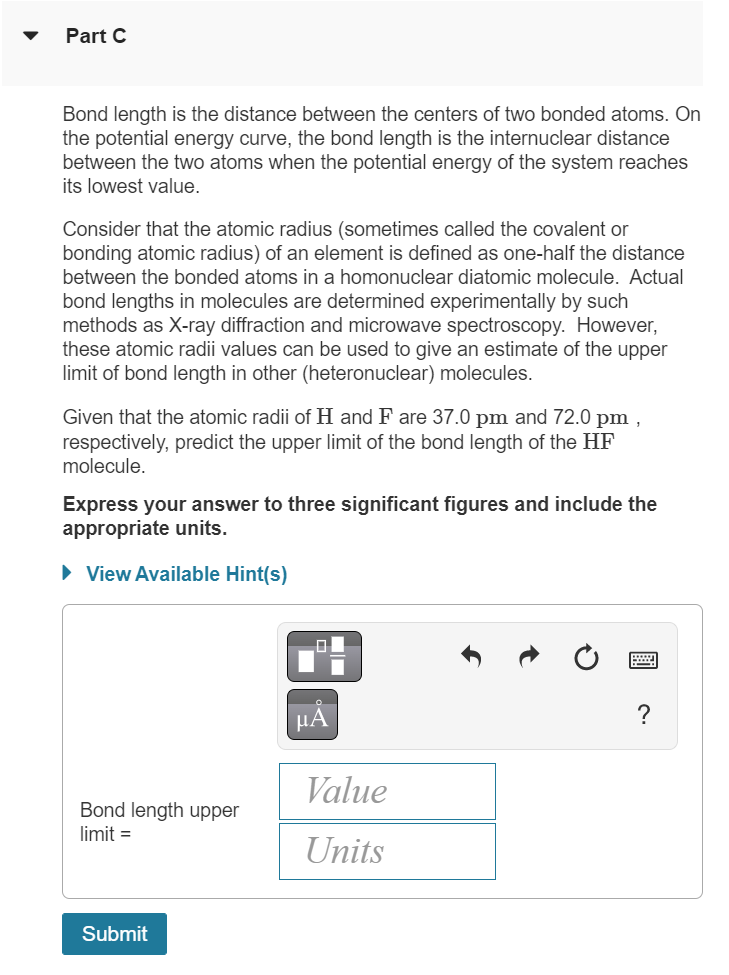
Transcribed Image Text:Part C
Bond length is the distance between the centers of two bonded atoms. On
the potential energy curve, the bond length is the internuclear distance
between the two atoms when the potential energy of the system reaches
its lowest value.
Consider that the atomic radius (sometimes called the covalent or
bonding atomic radius) of an element is defined as one-half the distance
between the bonded atoms in a homonuclear diatomic molecule. Actual
bond lengths in molecules are determined experimentally by such
methods as X-ray diffraction and microwave spectroscopy. However,
these atomic radii values can be used to give an estimate of the upper
limit of bond length in other (heteronuclear) molecules.
Given that the atomic radii of H and F are 37.0 pm and 72.0 pm,
respectively, predict the upper limit of the bond length of the HF
molecule.
Express your answer to three significant figures and include the
appropriate units.
► View Available Hint(s)
Bond length upper
limit=
Submit
μÅ
Value
Units
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning
