Part C ICN One o bond forms between a hybrid sp³ orbital on C and a p orbital on I; one o bond forms between a hybrid sp? orbital on C and a p orbital on N. One o bond forms between a hybrid sp² orbital on C and a p orbital on I; one o bond forms between a hybrid sp orbital on C and a p orbital on N. One o bond forms between a hybrid sp² orbital on C and a p orbital on I; one o bond forms between a hybrid sp° orbital on C and a p orbital on N. One o bond forms between a hybrid sp orbital on C and a p orbital on I; one o bond forms between a hybrid sp orbital on C and a p orbital on N.
Part C ICN One o bond forms between a hybrid sp³ orbital on C and a p orbital on I; one o bond forms between a hybrid sp? orbital on C and a p orbital on N. One o bond forms between a hybrid sp² orbital on C and a p orbital on I; one o bond forms between a hybrid sp orbital on C and a p orbital on N. One o bond forms between a hybrid sp² orbital on C and a p orbital on I; one o bond forms between a hybrid sp° orbital on C and a p orbital on N. One o bond forms between a hybrid sp orbital on C and a p orbital on I; one o bond forms between a hybrid sp orbital on C and a p orbital on N.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter9: Bonding And Molecular Structure: Orbital Hybridization And Molecular Orbitals
Section: Chapter Questions
Problem 71SCQ: Bromine forms a number of oxides of varying stability. (a) One oxide has 90.90% Br and 9.10% O....
Related questions
Question
Please answer question 20 Part A, B, and C

Transcribed Image Text:Part C
ICN
One o bond forms between a hybrid sp3 orbital on C and a p orbital on I; one o bond forms between a hybrid
sp? orbital on C and a
orbital on N.
One o bond forms between a hybrid sp orbital on C and a p orbital on I; one o bond forms between a hybrid
sp orbital on C and a p orbital on N.
One o bond forms between a hybrid sp orbital on C and a p orbital on I; one o bond forms between a hybrid
sp° orbital on C and a p orbital on N.
One o bond forms between a hybrid sp orbital on C and a p orbital on I; one o bond forms between a hybrid
sp orbital on C and a p orbital on N.
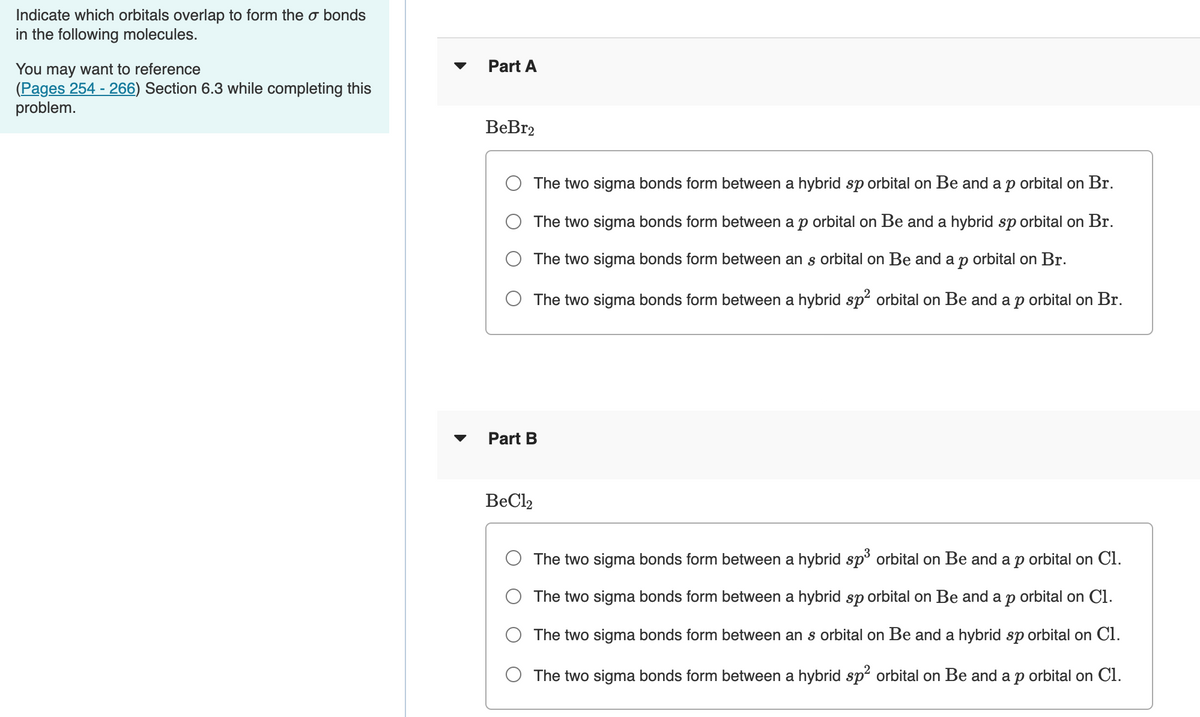
Transcribed Image Text:Indicate which orbitals overlap to form the o bonds
in the following molecules.
Part A
You may want to reference
(Pages 254 - 266) Section 6.3 while completing this
problem.
BeBr2
The two sigma bonds form between a hybrid sp orbital on Be and a p orbital on Br.
The two sigma bonds form between a p orbital on Be and a hybrid sp orbital on Br.
The two sigma bonds form between an s orbital on Be and a p orbital on Br.
O The two sigma bonds form between a hybrid sp? orbital on Be and ap orbital on Br.
Part B
BeCl2
The two sigma bonds form between a hybrid sp3 orbital on Be and a p orbital on Cl.
The two sigma bonds form between a hybrid sp orbital on Be and a p orbital on Cl.
The two sigma bonds form between an s orbital on Be and a hybrid sp orbital on Cl.
The two sigma bonds form between a hybrid sp orbital on Be and a p orbital on Cl.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning