Part C In the PhET simulation, make sure that the checkbox Stable/Unstable in the bottom right is checked. Using the PhET simulation as a reference, determine whether the following combinations of protons and neutrons form stable or unstable nuclei by adding protons and neutrons to the nucleus. Unstable nuclei will shake. If the Stable/Unstable checkbox in the lower right is also checked, the nuclei will be labeled Stable or Unstable. Sort the following combinations of protons and neutrons according to whether or not they form stable or unstable nuclei. Drag the appropriate items to their appropriate bins. ► View Available Hint(s) 2 protons and 0 neutrons Stable proton and 0 neutrons 4 protons and 5 neutrons 1 proton and 2 neutrons protons and 7 neutrons Unstable 6 protons and 5 neutrons 8 protons and 7 neutrons Reset 9 protons and 9 neutrons Help
Part C In the PhET simulation, make sure that the checkbox Stable/Unstable in the bottom right is checked. Using the PhET simulation as a reference, determine whether the following combinations of protons and neutrons form stable or unstable nuclei by adding protons and neutrons to the nucleus. Unstable nuclei will shake. If the Stable/Unstable checkbox in the lower right is also checked, the nuclei will be labeled Stable or Unstable. Sort the following combinations of protons and neutrons according to whether or not they form stable or unstable nuclei. Drag the appropriate items to their appropriate bins. ► View Available Hint(s) 2 protons and 0 neutrons Stable proton and 0 neutrons 4 protons and 5 neutrons 1 proton and 2 neutrons protons and 7 neutrons Unstable 6 protons and 5 neutrons 8 protons and 7 neutrons Reset 9 protons and 9 neutrons Help
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU1: Alchemy: Matter, Atomic Structure, And Bonding
SectionU1.16: Old Gold: Formation Of Elements
Problem 6E
Related questions
Question
Solve correctly please, be sure with some explanation also
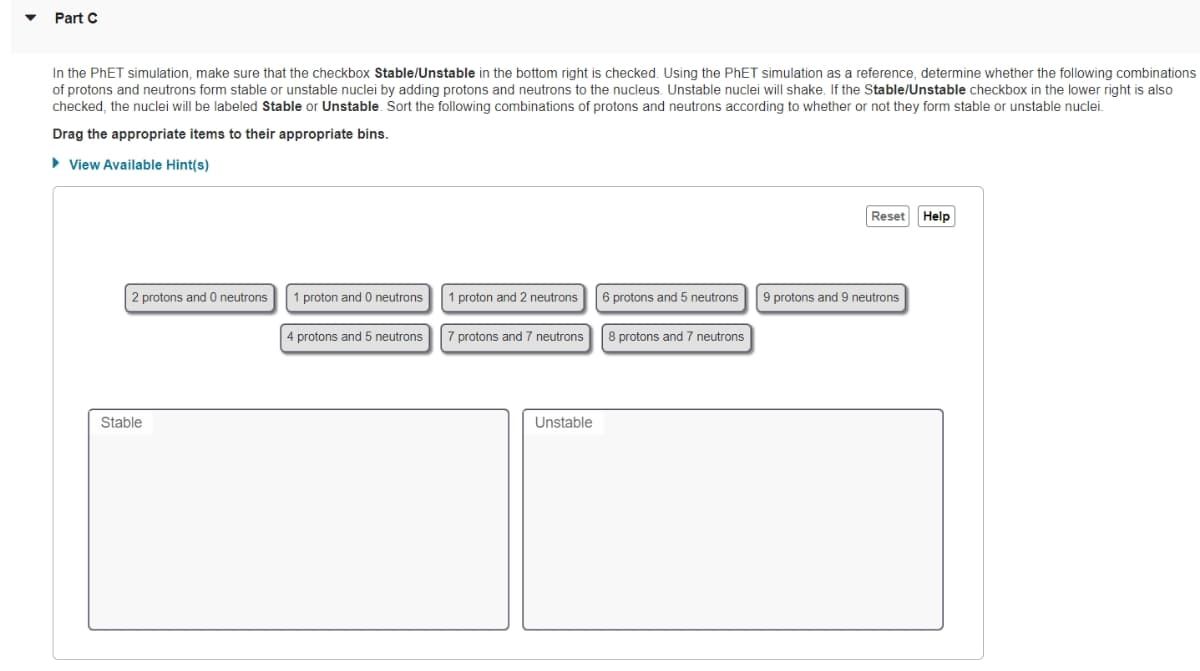
Transcribed Image Text:Part C
In the PhET simulation, make sure that the checkbox Stable/Unstable in the bottom right is checked. Using the PhET simulation as a reference, determine whether the following combinations
of protons and neutrons form stable or unstable nuclei by adding protons and neutrons to the nucleus. Unstable nuclei will shake. If the Stable/Unstable checkbox in the lower right is also
checked, the nuclei will be labeled Stable or Unstable. Sort the following combinations of protons and neutrons according to whether or not they form stable or unstable nuclei.
Drag the appropriate items to their appropriate bins.
► View Available Hint(s)
2 protons and 0 neutrons
Stable
proton and 0 neutrons
4 protons and 5 neutrons
1 proton and 2 neutrons
protons and 7 neutrons
Unstable
6 protons and 5 neutrons
8 protons and 7 neutrons
Reset
9 protons and 9 neutrons
Help
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning