Part D 1.40 Compare your answer for part A with the speed in air at the same temperature. The ratio of heat capacities for air is να ΑΣφ 1314.8 Vair Previous Answers Request Answer Submit Incorrect; Try Again; 2 attempts remaining Part E Compare your answer for part B with the speed in air at the same temperature. The ratio of heat capacities for air is 1.40 ΑΣφ atr Submit Request Answer Part F 1.40 Compare your answer for part C with the speed in air at the same temperature. The ratio of heat capacities for air is ΑΣφ ? atr Request Answer Submit Part A At a temperature of 27°C, what is the speed of longitudinal waves in hydrogen (molar mass 2.02 g/mol)? The ratio of heat capacities for hydrogen is y 1.41 v1320 m/s Previous Answers Submit Correct Part B At a temperature of 27 °C, what is the speed of longitudinal waves in helium (molar mass 4.00 g/mol)? The ratio of heat capacities for helium is y 1.67 v 1020 m/s Previous Answers Submit Correct Part C At a temperature of 27°C, what is the speed of longitudinal waves in argon (molar mass 39.9 g/mol)? The ratio of heat capacities for argon is y= 1.67 v323 m/s Previous Answers Submit Correct
Part D 1.40 Compare your answer for part A with the speed in air at the same temperature. The ratio of heat capacities for air is να ΑΣφ 1314.8 Vair Previous Answers Request Answer Submit Incorrect; Try Again; 2 attempts remaining Part E Compare your answer for part B with the speed in air at the same temperature. The ratio of heat capacities for air is 1.40 ΑΣφ atr Submit Request Answer Part F 1.40 Compare your answer for part C with the speed in air at the same temperature. The ratio of heat capacities for air is ΑΣφ ? atr Request Answer Submit Part A At a temperature of 27°C, what is the speed of longitudinal waves in hydrogen (molar mass 2.02 g/mol)? The ratio of heat capacities for hydrogen is y 1.41 v1320 m/s Previous Answers Submit Correct Part B At a temperature of 27 °C, what is the speed of longitudinal waves in helium (molar mass 4.00 g/mol)? The ratio of heat capacities for helium is y 1.67 v 1020 m/s Previous Answers Submit Correct Part C At a temperature of 27°C, what is the speed of longitudinal waves in argon (molar mass 39.9 g/mol)? The ratio of heat capacities for argon is y= 1.67 v323 m/s Previous Answers Submit Correct
Related questions
Question
I don't understand what the problem is asking me for in part d and I've tried different ways to compare it and keep getting it wrong.
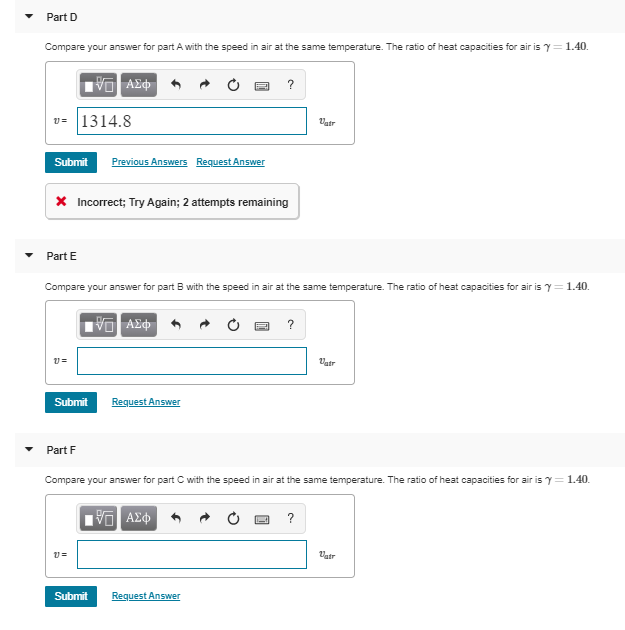
Transcribed Image Text:Part D
1.40
Compare your answer for part A with the speed in air at the same temperature. The ratio of heat capacities for air is
να ΑΣφ
1314.8
Vair
Previous Answers Request Answer
Submit
Incorrect; Try Again; 2 attempts remaining
Part E
Compare your answer for part B with the speed in air at the same temperature. The ratio of heat capacities for air is
1.40
ΑΣφ
atr
Submit
Request Answer
Part F
1.40
Compare your answer for part C with the speed in air at the same temperature. The ratio of heat capacities for air is
ΑΣφ
?
atr
Request Answer
Submit
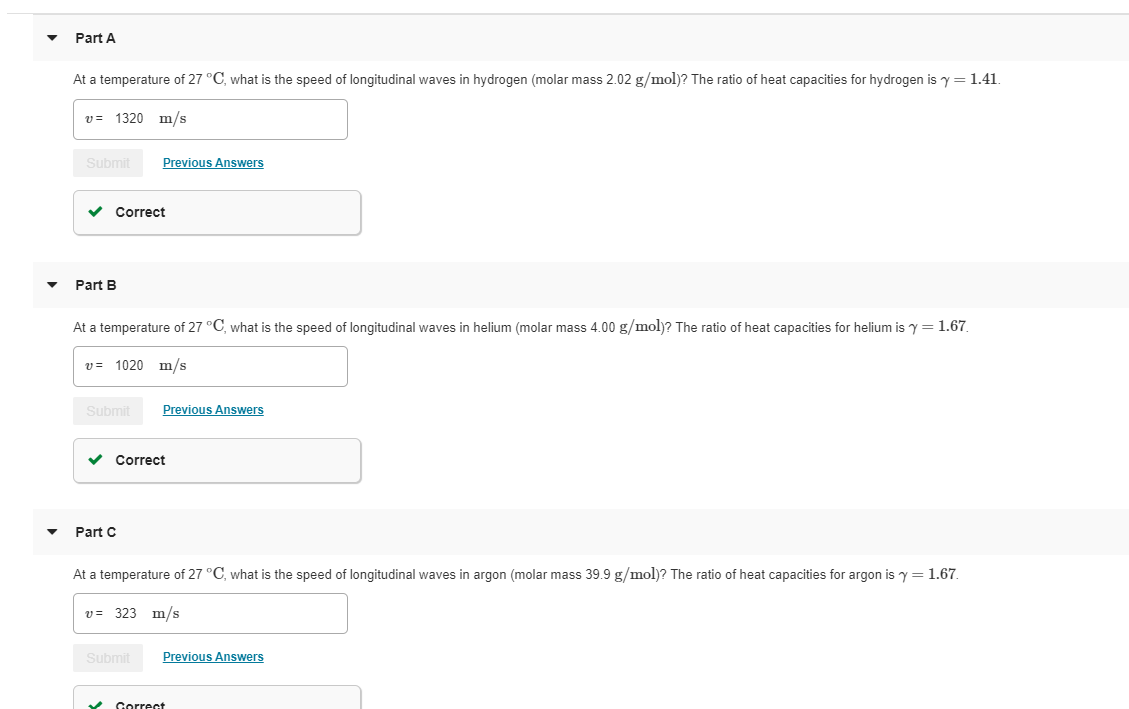
Transcribed Image Text:Part A
At a temperature of 27°C, what is the speed of longitudinal waves in hydrogen (molar mass 2.02 g/mol)? The ratio of heat capacities for hydrogen is y
1.41
v1320 m/s
Previous Answers
Submit
Correct
Part B
At a temperature of 27 °C, what is the speed of longitudinal waves in helium (molar mass 4.00 g/mol)? The ratio of heat capacities for helium is y
1.67
v 1020 m/s
Previous Answers
Submit
Correct
Part C
At a temperature of 27°C, what is the speed of longitudinal waves in argon (molar mass 39.9 g/mol)? The ratio of heat capacities for argon is y= 1.67
v323 m/s
Previous Answers
Submit
Correct
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images
