Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section5.8: Product- Or Reactant-favored Reactions And Thermodynamics
Problem 1.2ACP
Related questions
Question
Transcribed Image Text:7
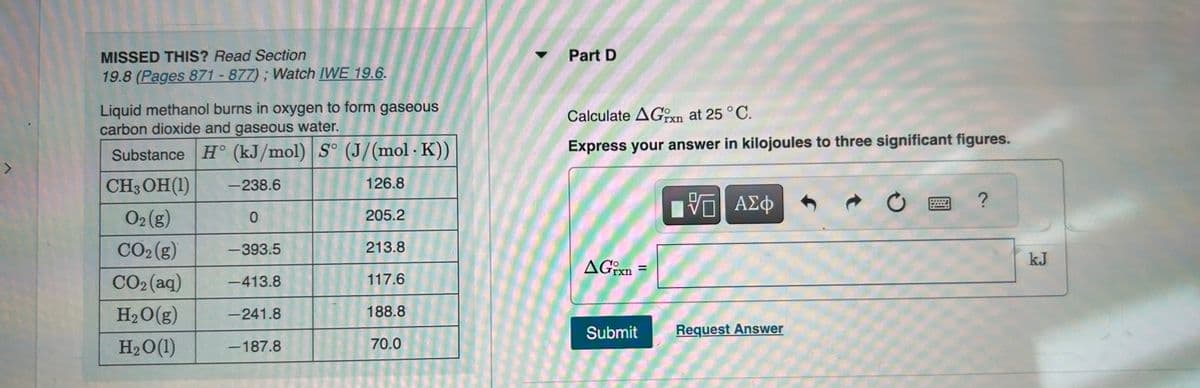
MISSED THIS? Read Section
19.8 (Pages 871-877); Watch IWE 19.6.
Liquid methanol burns in oxygen to form gaseous
carbon dioxide and gaseous water.
Substance H (kJ/mol) S° (J/(mol. K))
CH3OH (1)
-238.6
126.8
O2 (g)
0
CO₂(g)
-393.5
CO2(aq) -413.8
H₂O(g) -241.8
H₂O(1)
-187.8
205.2
213.8
117.6
188.8
70.0
Part D
Calculate AGxn at 25 °C.
Express your answer in kilojoules to three significant figures.
AGixn=
Submit
VE ΑΣΦ
VO
Request Answer
?
kJ
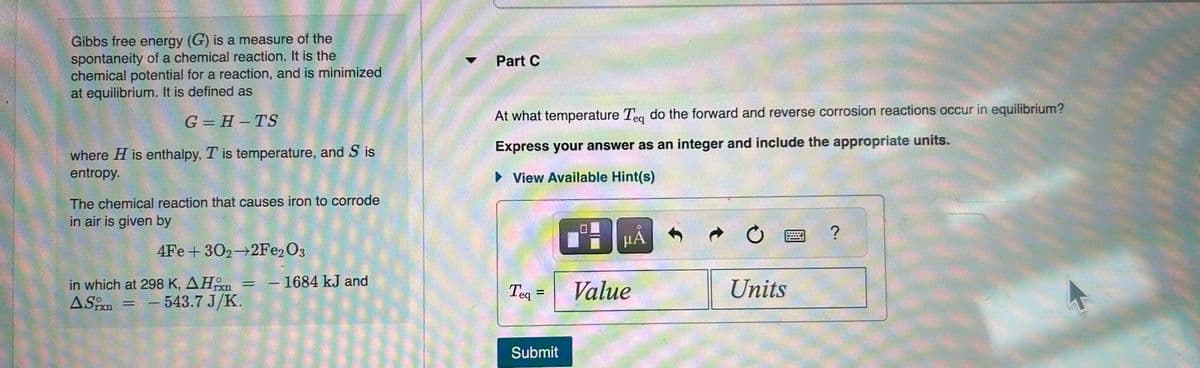
Transcribed Image Text:Gibbs free energy (G) is a measure of the
spontaneity of a chemical reaction. It is the
chemical potential for a reaction, and is minimized
at equilibrium. It is defined as
G=H-TS
where H is enthalpy, T is temperature, and S is
entropy.
The chemical reaction that causes iron to corrode
in air is given by
4Fe +302-2Fe2O3
in which at 298 K, AH = - 1684 kJ and
ASixn
- 543.7 J/K.
=
Part C
At what temperature Teq do the forward and reverse corrosion reactions occur in equilibrium?
Express your answer as an integer and include the appropriate units.
► View Available Hint(s)
Teq
=
Submit
14
μÅ
Value
Units
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax