Part D Follow these steps to complete the table. 1. Reuse the same test tubes from part C, labeled Fe2* and Fe*. Be sure they're clean. 2. Add 4 milliliters of iron(lI) sulfate to the test tube labeled Fe2+. 3. Add 4 milliliters of iron(II) nitrate to the test tube labeled Fe3+. 4. Add 4 milliliters of sodium hydroxide to each test tube. 5. Observe the contents of the test tubes for about 10 minutes. Record your observations, noting evidence of a chemical reaction. If there is no evidence of a reaction, write "no reaction." x' X, Font Sizes 三 E 星 -三ヨ 用, Substances Mixed Iron lon Present Description of the Reaction iron(II) sulfate and sodium hydroxide iron(II) (Fe2t) iron(III) nitrate and sodium hydroxide iron(III) (Fe)
Part D Follow these steps to complete the table. 1. Reuse the same test tubes from part C, labeled Fe2* and Fe*. Be sure they're clean. 2. Add 4 milliliters of iron(lI) sulfate to the test tube labeled Fe2+. 3. Add 4 milliliters of iron(II) nitrate to the test tube labeled Fe3+. 4. Add 4 milliliters of sodium hydroxide to each test tube. 5. Observe the contents of the test tubes for about 10 minutes. Record your observations, noting evidence of a chemical reaction. If there is no evidence of a reaction, write "no reaction." x' X, Font Sizes 三 E 星 -三ヨ 用, Substances Mixed Iron lon Present Description of the Reaction iron(II) sulfate and sodium hydroxide iron(II) (Fe2t) iron(III) nitrate and sodium hydroxide iron(III) (Fe)
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter24: Nuclear Chemistry
Section24.2: Radioactive Decay
Problem 11PP
Related questions
Question
100%
![Activity: Charges on Transition Metal lons
5 of 6
Part C
Follow these steps to complete the table:
1. Reuse the same test tubes from part B, labeled Fe2* and Fe3+. Be sure they're clean.
2. Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe2*.
3. Add 4 milliliters of iron(III) nitrate to the test tube labeled Fe3+.
4. Add 4 milliliters of potassium permanganate to each test tube.
5. Observe the contents of the test tubes for about 10 minutes. Record your observations, noting any
evidence of a chemical reaction. If there is no evidence of a reaction, write "no reaction."
BIU
x
Font Sizes
三三 国E 三 三 v田。
Substances Mixed
Iron lon Present
Description of the Reaction
WHen pale green irón (II) sulfate is mixed with potassium
permanganate (KMN04), then Fe* will seduce pink color in Mn04 to
colorless Mn2+ and itself will outside to yellow color Fe3+.
iron(II) sulfate and potassium permanganate
iron(II) (Fe2+)
iron(III) nitrate and potassium permanganate
iron(III) (Fe3+
There is no section between Iron (III) nitrate [FE9NO-] KMNO4.
Characters used: 435 / 15000](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ff1d1ee19-48b1-40f4-831a-39741af7f233%2F2c7cbf95-b571-497a-9ef7-b1bbf30bd3d7%2Fp6sksf_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Activity: Charges on Transition Metal lons
5 of 6
Part C
Follow these steps to complete the table:
1. Reuse the same test tubes from part B, labeled Fe2* and Fe3+. Be sure they're clean.
2. Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe2*.
3. Add 4 milliliters of iron(III) nitrate to the test tube labeled Fe3+.
4. Add 4 milliliters of potassium permanganate to each test tube.
5. Observe the contents of the test tubes for about 10 minutes. Record your observations, noting any
evidence of a chemical reaction. If there is no evidence of a reaction, write "no reaction."
BIU
x
Font Sizes
三三 国E 三 三 v田。
Substances Mixed
Iron lon Present
Description of the Reaction
WHen pale green irón (II) sulfate is mixed with potassium
permanganate (KMN04), then Fe* will seduce pink color in Mn04 to
colorless Mn2+ and itself will outside to yellow color Fe3+.
iron(II) sulfate and potassium permanganate
iron(II) (Fe2+)
iron(III) nitrate and potassium permanganate
iron(III) (Fe3+
There is no section between Iron (III) nitrate [FE9NO-] KMNO4.
Characters used: 435 / 15000
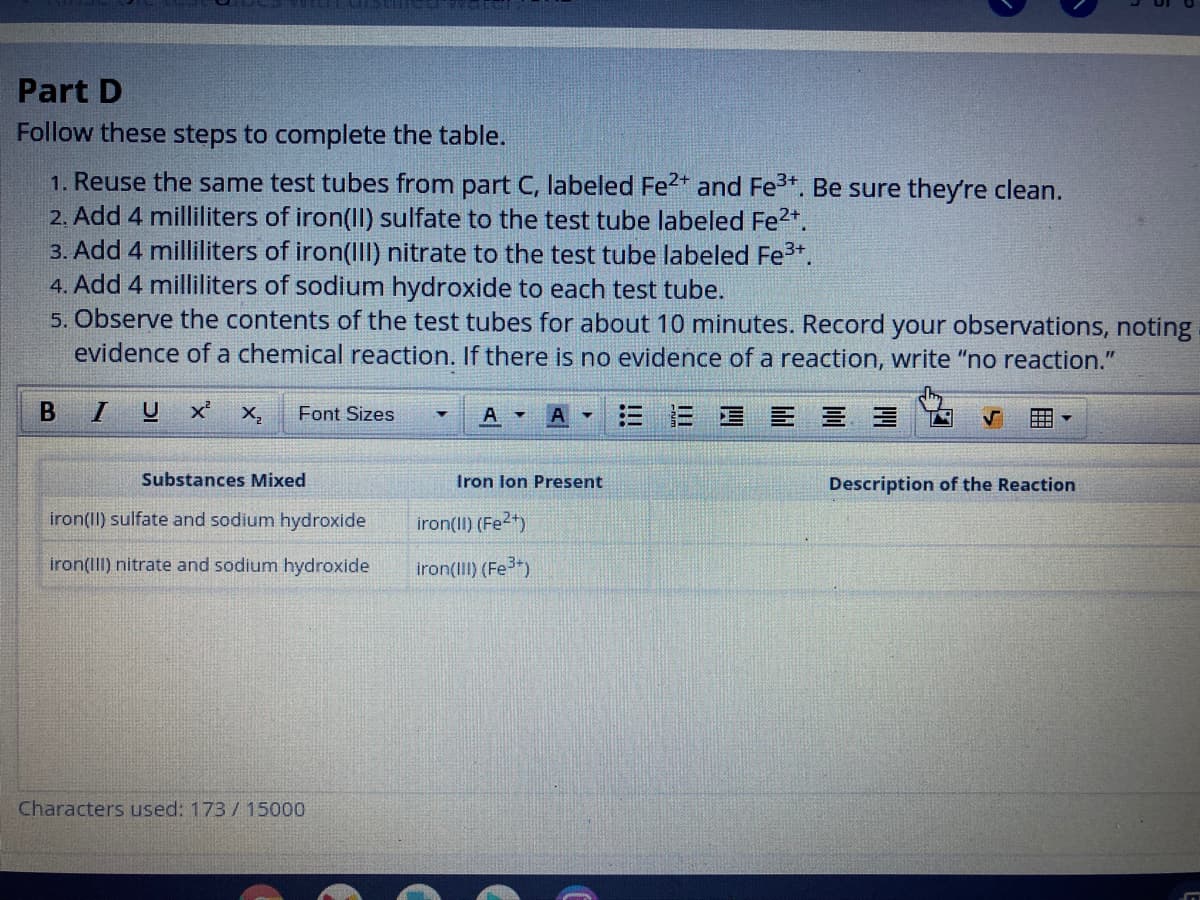
Transcribed Image Text:Part D
Follow these steps to complete the table.
1. Reuse the same test tubes from part C, labeled Fe2* and Fe3+. Be sure they're clean.
2. Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe2*.
3. Add 4 milliliters of iron(III) nitrate to the test tube labeled Fe3+.
4. Add 4 milliliters of sodium hydroxide to each test tube.
5. Observe the contents of the test tubes for about 10 minutes. Record your observations, noting
evidence of a chemical reaction. If there is no evidence of a reaction, write "no reaction."
B
X,
Font Sizes
三 三 =
田
Substances Mixed
Iron lon Present
Description of the Reaction
iron(II) sulfate and sodium hydroxide
iron(II) (Fe2)
iron(III) nitrate and sodium hydroxide
iron(III) (Fe)
Characters used: 173/15000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co
