A chemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide solution. He has a 0.1585 M standard sodium hydroxide solution. He takes a 25.00 mL sample of the original acid solutio and dilutes it to 250.0 mL. Then, he takes a 10.00 mL sample of the dilute acid solution and titrates it with the standard solution. The endpoint was reached after the addition of 11.32 mL of the standard solution. What is the concentration of the original sulfuric acid solution? concentration: M
A chemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide solution. He has a 0.1585 M standard sodium hydroxide solution. He takes a 25.00 mL sample of the original acid solutio and dilutes it to 250.0 mL. Then, he takes a 10.00 mL sample of the dilute acid solution and titrates it with the standard solution. The endpoint was reached after the addition of 11.32 mL of the standard solution. What is the concentration of the original sulfuric acid solution? concentration: M
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 98AP
Related questions
Question
100%
Help
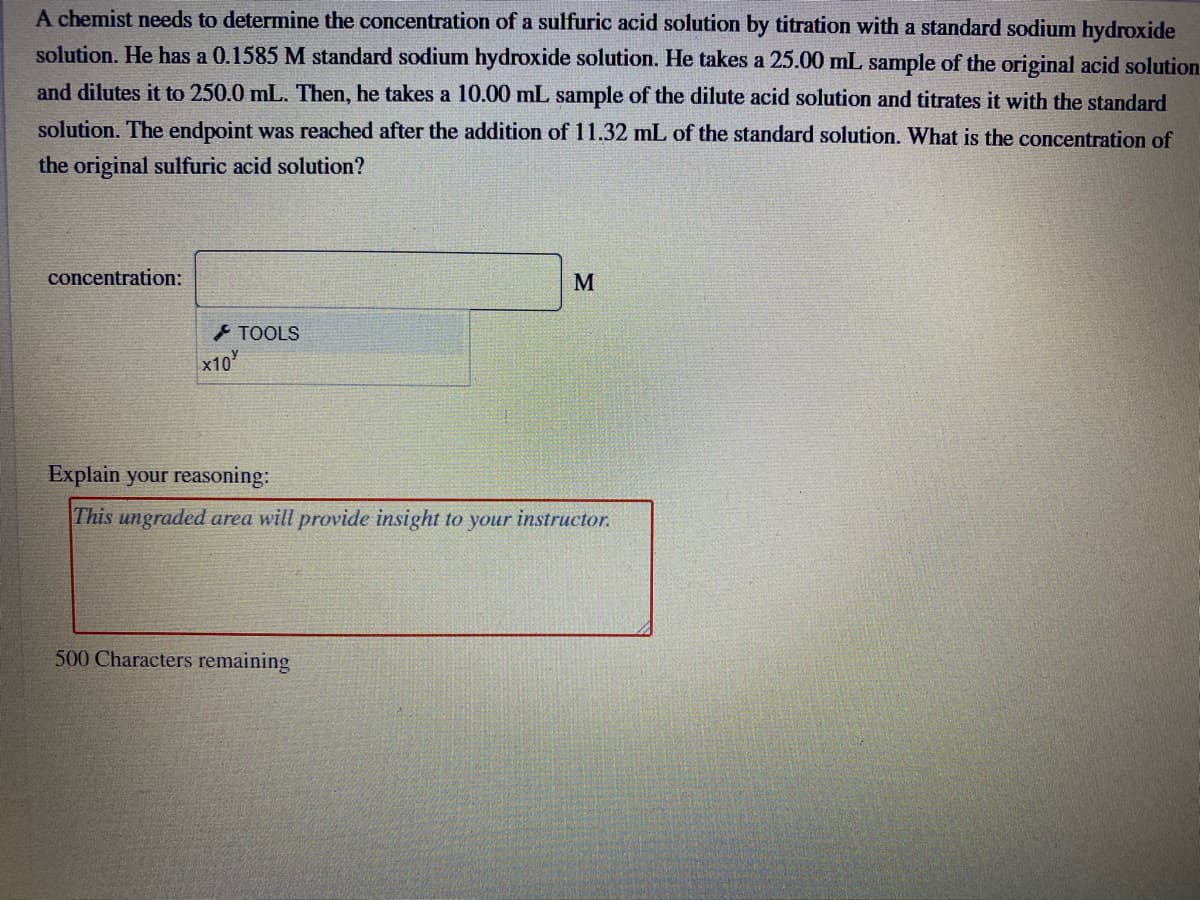
Transcribed Image Text:A chemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide
solution. He has a 0.1585 M standard sodium hydroxide solution. He takes a 25.00 mL sample of the original acid solution
and dilutes it to 250.0 mL. Then, he takes a 10.00 mL sample of the dilute acid solution and titrates it with the standard
solution. The endpoint was reached after the addition of 11.32 mL of the standard solution. What is the concentration of
the original sulfuric acid solution?
concentration:
M
TOOLS
x10
Explain your reasoning:
This ungraded area will provide insight to your instructor.
500 Characters remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning