Part I. Cause and Effect: lIdentify the effect of the condition on the indicated parameter. Write increase, decrease, or no effect, and provide an explanation (3 sentences maximum). Assume you performed the experiment as stated in the laboratory manual. A. The test tube used in the determination of the AHn for nitric acid was still wet with water. [magnitude of AHxn 'rxn B. The actual concentration of the sodium hydroxide used in the calibration part of the experiment was lower than the stated value. [Ccal C. The actual concentration of the sodium hydroxide used in the calibration part of the experiment was lower than the stated value. However, the NaOH solution used in the determination of AHn was of the correct concentration. [magnitude of ΔΗ 'rxn
Part I. Cause and Effect: lIdentify the effect of the condition on the indicated parameter. Write increase, decrease, or no effect, and provide an explanation (3 sentences maximum). Assume you performed the experiment as stated in the laboratory manual. A. The test tube used in the determination of the AHn for nitric acid was still wet with water. [magnitude of AHxn 'rxn B. The actual concentration of the sodium hydroxide used in the calibration part of the experiment was lower than the stated value. [Ccal C. The actual concentration of the sodium hydroxide used in the calibration part of the experiment was lower than the stated value. However, the NaOH solution used in the determination of AHn was of the correct concentration. [magnitude of ΔΗ 'rxn
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.8QAP
Related questions
Question
Hi! I need answers on these three situations. Thank youuuu! Please.
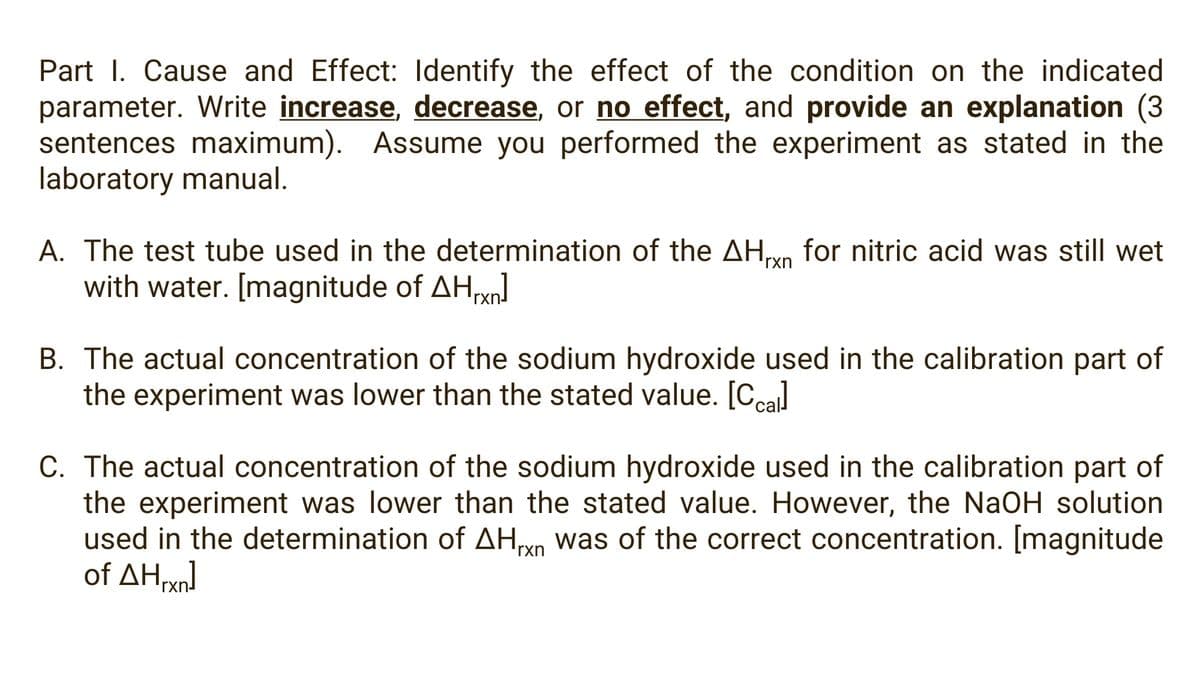
Transcribed Image Text:Part I. Cause and Effect: Identify the effect of the condition on the indicated
parameter. Write increase, decrease, or no effect, and provide an explanation (3
sentences maximum). Assume you performed the experiment as stated in the
laboratory manual.
A. The test tube used in the determination of the AHvn for nitric acid was still wet
with water. [magnitude of AHxn
rxn
B. The actual concentration of the sodium hydroxide used in the calibration part of
the experiment was lower than the stated value. [Ccal
C. The actual concentration of the sodium hydroxide used in the calibration part of
the experiment was lower than the stated value. However, the NaOH solution
used in the determination of AHyn was of the correct concentration. [magnitude
of ΔΗΜη
rxn
rxn
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole
