Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter16: Reactions Between Acids And Bases
Section: Chapter Questions
Problem 16.54QE
Related questions
Question
Please check if my second data table is correct. I don't think the molar mass is correct
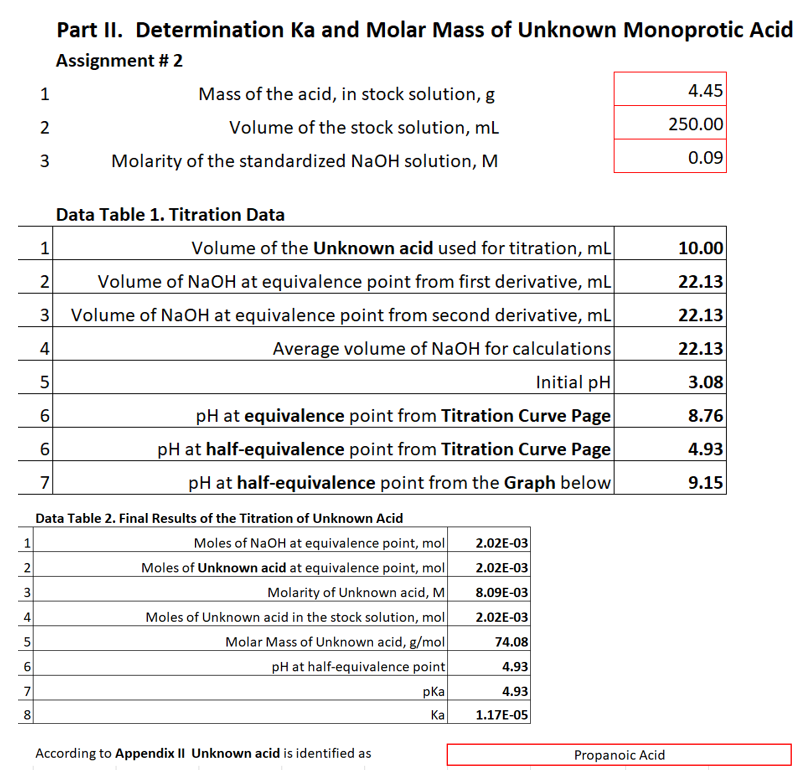
Transcribed Image Text:Part II. Determination Ka and Molar Mass of Unknown Monoprotic Acid
Assignment # 2
1
Mass of the acid, in stock solution, g
4.45
2
Volume of the stock solution, ml
250.00
3
Molarity of the standardized NaOH solution, M
0.09
Data Table 1. Titration Data
1
Volume of the Unknown acid used for titration, mL
10.00
Volume of NaOH at equivalence point from first derivative, ml
3 Volume of NaOH at equivalence point from second derivative, mL
2
22.13
22.13
4
Average volume of NaOH for calculations
22.13
Initial pH
3.08
6
pH at equivalence point from Titration Curve Page
8.76
6
pH at half-equivalence point from Titration Curve Page
4.93
7
pH at half-equivalence point from the Graph below
9.15
Data Table 2. Final Results of the Titration of Unknown Acid
Moles of NaOH at equivalence point, mol|
Moles of Unknown acid at equivalence point, mol
Molarity of Unknown acid, M
Moles of Unknown acid in the stock solution, mol
Molar Mass of Unknown acid, g/mol
pH at half-equivalence point
2.02E-03
2.02E-03
8.09E-03
2.02E-03
74.08
3
4
4.93
7
pKa
4.93
8
Ка
1.17E-05
According to Appendix II Unknown acid is identified as
Propanoic Acid
2.
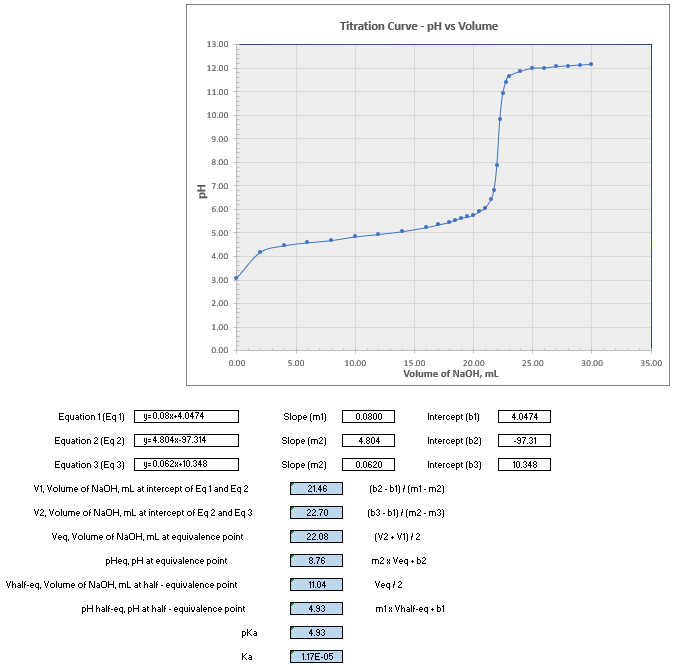
Transcribed Image Text:Titration Curve - pH vs Volume
13.00
12.00
11.00
10.00
9.00
8.00
7.00
6.00
5.00
4.00
3.00
2.00
1.00
0.00
0.00
5.00
10.00
15.00
20.00
25.00
30.00
35.00
Volume of NaOH, ml
Equation 1 (Eq 1)
y= 0.08x+4.0474
Slope (ml)
0.0800
Intercept (b1)
4.0474
Equation 2 (Eq 2)
y= 4.804x-97.314
Slope (m2)
4.804
Intercept (b2)
-97.31
Equation 3 (Eq 3)
y=0.062x-10.348
Slope (m2)
0.0620
Intercept (b3)
10.348
V1, Volume of NAOH, ml at intercept of Eq 1 and Eq 2
21.46
(b2 - b1) (m1 - m2)
v2, Volume of NAOH, ml at intercept of Eq 2 and Eq 3
22.70
(b3 - b1) (m2 - m3)
Veg, Volume of NAOH, mL at equivalence point
(V2 • V1) /2
22.08
pHeq, pH at equivalence point
8.76
m2 x Veq • b2
Vhalf-eq, Volume of NaOH, ml at half - equivalence point
11.04
Veq 12
pH half-eq, pH at half - equivalence point
4.93
m1x Vhalf-eq • b1
pKa
4.93
Ка
1.17E-05
Hd
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 6 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

