Use the References to access important values if needed for this question. amino acid glycine has the molecular formula C,H&NO,, and the amino acid isoleucine has the molecular formula CH13NO, ermine how many moles of isoleucine contain the same number of atoms as 1.97 mol of glycine. moles Submit Answer 5 question attempts remaining
Use the References to access important values if needed for this question. amino acid glycine has the molecular formula C,H&NO,, and the amino acid isoleucine has the molecular formula CH13NO, ermine how many moles of isoleucine contain the same number of atoms as 1.97 mol of glycine. moles Submit Answer 5 question attempts remaining
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter16: Amines And Amides
Section: Chapter Questions
Problem 16.67E
Related questions
Question
Transcribed Image Text:DTAP
Q Search
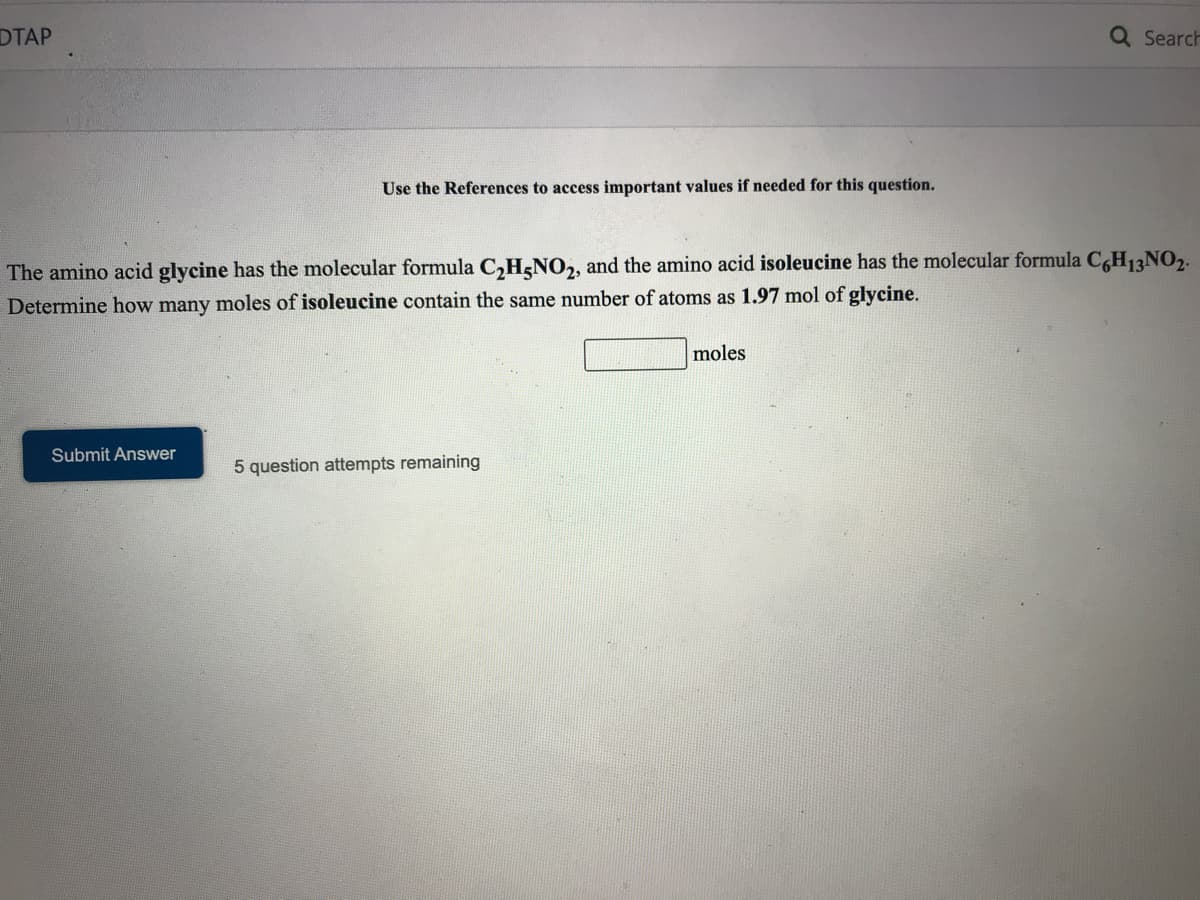
Use the References to access important values if needed for this question.
The amino acid glycine has the molecular formula C,H<NO2, and the amino acid isoleucine has the molecular formula CH,13NO,.
Determine how many moles of isoleucine contain the same number of atoms as 1.97 mol of glycine.
moles
Submit Answer
5 question attempts remaining
Expert Solution
Step 1
The question is based on mole concept.
first we calculate number of atoms of glycine present in given moles.
then we equated these atoms to the atoms of isoleucine and consequently calculated number of moles of isoleucine should be there.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning