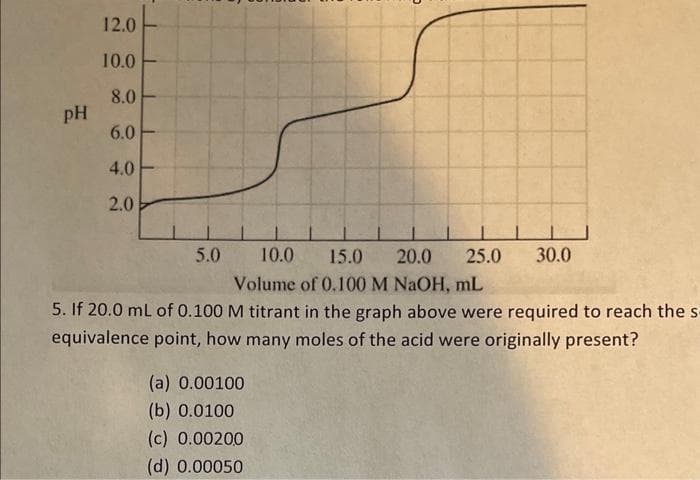
pH 12.0 10.0 8.0 6.0 4.0 2.0 10.0 Volume of 0.100 M NaOH, mL 5. If 20.0 mL of 0.100 M titrant in the graph above were required to reach the s equivalence point, how many moles of the acid were originally present? 5.0 (a) 0.00100 (b) 0.0100 (c) 0.00200 (d) 0.00050 15.0 20.0 25.0 30.0
Q: Which of these chemical equations does not describe a redox reaction? Show the oxidation states of…
A: 1. Graham's law of effusion states that the rate of effusion of a gas is inversely proportional to…
Q: Which atom has the largest atomic radius? Ge As Se Br Kr Ge As Se…
A:
Q: Macmillan Learning Write the balanced net ionic equation for the reactions that occur when the given…
A: To write a net ionic equation these are few points which we have to keep in mind :- 1 at first we…
Q: Which disaccharide is formed from glucose and fructose? 1 A
A: Answer - Sucrose
Q: Dilute the stock solution: Using the solutions in the burets, carefully measure the following…
A: Calculation of Molarity of Cu(NO3)2.6H2O :- Molarity is defined as the no of g-moles of solute…
Q: In step 1, where did you get the value of -2.303?
A: We have to tell from where the value -2.303 appeared. Introduction: Nernst equation.
Q: The value of Kp is 22,100 at 25°C for: 2 H₂(g) + CO₂(g) ≥ CH3OH (g) Predict the direction the…
A: Reaction quotient at constant pressure is the product of the partial pressure of the products, each…
Q: Chemistry 14) A 60.0 mL quantity of 0.80 M HCI is mixed with 60.0 mL of 0.80 M KOH in a constant…
A:
Q: What are possible units for the rate of a reaction? Multiple Choice Tu kes the World-CMS»EJ HEATHER
A:
Q: The heat of vaporization of ammonia is 21.7 kJ mol-¹ and the boiling point of ammonia is -33.3 °C.…
A:
Q: Decide whether the Lewis structure proposed for each molecule is reasonable or not. molecule SF4 CH…
A: we have to determine which of the given Lewis structures is reasonable
Q: What is the classification for a monosaccharide with three carbon atoms and a carbonyl group on the…
A: Correct answer - Trioses
Q: Write the balanced chemical equation between H2SO4 and KOH in aqueous solution. This is called a…
A:
Q: 1.065 g of stainless steel was dissolved in hydrochloric acid (chromium as Cr 3+ ) and diluted to…
A: Given ---> Stainless steel solution=1.065g Volume of water for dilution= 500ml At walder…
Q: Consider 15 mL of an aqueous solution containing 2.0 g of an organic solute. If the distribution…
A: Given:- Dissociation constant for water and ether= 20 Weight of solute= 2g Volume of ether used for…
Q: Give the name of the missing organic reactant in the following reaction. ? + 2 Ag(NH3)2+ + 3OH-…
A:
Q: entify the alkyne you would use to prepare the following ketone via acid-catalyzed hydration, and…
A:
Q: structure of the enolate anions and the corresponding resonance contributors, the related…
A: Here we are required to find the mechanism for the base catalysed aldol reaction.
Q: 4. Proton NMR C₂H12 8 5 7 6 1 5 4 3 Chemical Shift (ppm) 2 6 1
A: “Since you have asked multiple question, we will solve the first question for you. If you want any…
Q: What is the role of each reagent in the preparation of a-chloro-2,6-dimethylacetanilide?…
A: This is carbonyl addition reaction where -NH2 attacks on carbonyl of acid chloride to give acid…
Q: In one demonstration of this reaction, 33.00 mL of H₂ are collected over water at 25°C. Atmospheric…
A:
Q: On a 10 day wilderness expedition you'll need to heat 2.0 kg of water to the boiling point each day.…
A:
Q: The rate law predicted by the following two-step mechanism is Rate = K[A][B]. A C+B (slow) A+B → C+E…
A:
Q: A balloon that is filled with gas was released at ground level where the pressure was 745.0 mmHg an…
A: According to combined gas law, P1V1/T1 = P2V2/T2 Given P1 = 745.0 mm Hg V1 = 85.0 L T1 = 39.0°C = 85…
Q: Questions 5 & 6 both use the chemical reaction below: Cr3+ (aq) + SCN (aq) CrSCN²+ (aq) Solutions of…
A:
Q: 3. Given the data: 2 Cu(s) + S(s) → Cu₂S (s) AH = -79.5 kJ S (s) + O₂(g) → SO₂ (g) AH=-297 kJ Cu₂S…
A:
Q: A student wishes to determine the chloride ion concentration in a water sample at 25 °C using a…
A: Given, AgCl s + e- → Ag s + Cl- aq E°red = 0.2223 V…
Q: Concentration: 15.8mg/L Final volume: 100.0mL What is the concentration of the phosphate in the…
A:
Q: Using reaction free energy to predict equilibrium composition Consider the following equilibrium:…
A:
Q: Decide whether each pair of elements in the table below will form an ionic compound. If they will,…
A: Ionic compound are the compounds which are formed when the metal and non-metals reacts with each…
Q: Chemists can produce silver metal by reacting copper metal with a solution of silver nitrate: Cu(s)…
A: GivenCu(s) + 2 AgNO3(aq) → 2 Ag(s) + Cu(NO3)2 (aq)Moles of Cu initially = 0.24 molMoles of…
Q: 10 a) Write the major products of cis-2-butene with H+/H₂O b) Write the major products of…
A:
Q: QUESTION 13 :CO: Consider the provided Lewis structure for carbon monoxide: CO. Fill in the blanks…
A: The given molecule is carbon monoxide.
Q: A series of reactions is carried out in separate containers at a constant volume and a constant…
A: According to ideal gas law pV = nRT Where p = pressure V= volume n = mole of gas R= gas constant…
Q: An unknown gas effuses at a rate 0.667 times the rate of N₂. What is the molar mass of the unknown…
A:
Q: Follow the steps to calculate the concentration of the HCI solution. 1-Calculate the…
A: Here we are provided with limited data and hence we can answer it in terms of general values. This…
Q: Solids have a greater strength of attraction between its molecules than liquids True False
A: This is a basic question from states of matter. As we know, solids and liquids are two states of…
Q: Determine the missing concentration for the following voltaic cell at 25 °C Cr(s) | Cr³(aq, 0.0022…
A:
Q: SO3 ő Formula PH3 8 CO SO4²- SCN¹- Number of Valence Electrons Lewis Structure
A: we have to complete the given table for the Lewis structures of the given molecules and ions
Q: 1. a.)What is the pH of a buffer made by mixing 0.30M CH3NH2 and 0.36M CH3NH3Br? You must show all…
A: pH + pOH =14 pOH of basic buffer solution. pOH = pkb + log [salt/base] On addition of strong acid,…
Q: Which of the following is the correct equilibrium expression for the autoionization of water?…
A:
Q: How would you express this in the ratio of final temperature to initial temperature?
A: According to ideal gas law PV = nRT Here, P = pressure , V= volume, n = mole of gas , R= gas…
Q: How many moles of chlorine gas at 120.0 °C and 26.1 bar would occupy a vessel of 25.5 L? R = 0.08314…
A:
Q: For the reaction HNC(g) → HCN(g) If the initial concentration of HNC is 0.80 M, what is the…
A: The given equilibrium reaction is HNC (g) ⇆HCN (g); K = 8.0 The initial concentration of HNC is…
Q: .2 Calculate a value for the atomic radius of a chromium atom with a coordination number of 12 given…
A: Given, crystal of chromium ------> BCC unit cell Radius= 128pm Formula for atomic redius in…
Q: A 25.7 mL sample of liquid ethanol (C₂H₅OH, density = 0.789 g/mL) was injected into a 10.0 L…
A: Given, volume of sample of C₂H₅OH = 25.7 mL
Q: Draw all diastereomers and enantiomers for this compound.
A: Well the given compound has two chiral centers. So total number of stereoisomers is 2n=22=4 So we…
Q: draw the skeletal (line-bond) structure of (3R, 4R)-4-bromo-3-chloroheptane
A: Organic chemistry is branch of chemistry in which we deal with organic compounds. IUPAC nomenclature…
Q: Which of the following will have the lowest average kinetic energy? OA) H₂ at 400 °C O B) O₂ at 300…
A: 1) We have to tell among the following which one has the lowest average kinetic energy. H2 at 400°C…
Q: A 20.0 ml. sample of 0.0250 M Cu²+ buffered at pH 10.00 is titrated with 0.0200 M EDTA. Calculate…
A: A 20.00 mL solution of 0.0250 M Cu2+ solution buffered at a pH of 10.00 is titrated against a 0.0200…
Step by step
Solved in 2 steps

- Na2CO3 served as the primary standard in a titration experiment. Find the molarity of the titrant given the following data in 3 decimal places. Show solutions Primary Standard Used: Na2CO3Formula Mass of 1º standard: 105.99 g/mol% purity of 1º standard: 95% Trial 1 2 3 1º Standard weight, g 0.1005 0.1001 0.0997 Net volume of HCl, mL 9.30 9.00 8.90 Molarity of HCl X1 X2 X3Part A: Standardization of a Sodium Hydroxide Solution Titration 1 Titration 2 Titration 3 Mass of 125 mL flask 45.849g 46.715g 44.953g Mass of flask and KHP 46.849g 47.745g 46.003g Initial buret reading (mL) 0.5 ml 0.5 ml 0.5 ml Final buret reading (mL) 27.8 ml 26.5 ml 26.7 ml Volume of NaOH used (mL) 45.11 ml 45.06 ml 45.14 ml Calculations Titration 1 Titration 2 Titration 3 Moles of KHP Moles of NaOH Molarity of NaOH Average Molarity of NaOH: _______________While preparing the samples for analysis, you forgot that you already added phenolphthalein indicator so you added some more. What will be its effect in the volume of the titrant needed to reach the endpoint? Select one: A. Increase B. Decrease C. No effect D. Cannot be determined Primary standards are used to determine the exact concentration of the titrant. Based on the criteria of primary standards, which of the following cannot be used as a primary standard? Select one: A.Oxalic acid B. Sodium hydroxide C. Sodium carbonate D. Potassium dichromate During titration, you notice that the retention of the faint pink color is longer than earlier. Your lab partner suggested that you add a “half-drop” to prevent over-titration of the analyte solution. Is it ethical to do the “half-drop” technique? Select one: A. NO. It will cause error in calculations if you forgot to record the volume reading before adding the half-drop. B. YES. No error in calculations will be encountered provided…
- Anachem Titration curved Calculate the pH of the solution for every 0.1 ml of the titrant (from 0 mL to 30 mL) and generate a titration curve using Excel. Highlight each stage in the titration and the equivalence point and obtain the first derivative of the curve (that is ΔpH / Δvolume). 1. A 25 mL solution of 0.2521 M HCl was titrated with 0.3133 M NaOH 2. A 25 mL solution of 0.4153 M NaOH was titrated with 0.5191 M HNO3 3. A 10 mL solution of 0.0543 M formic acid was titrated with 0.0332 M KOHLimestone consists mainly of the mineral calcite, CaCO3. The carbonate content of a 0.5972 g sample of powdered limestone was measured by suspending the powder in water, adding 25.00 mL of 0.508 molar hydrochloric acid, and heating to dissolve the solid and dispel carbon dioxide gas. The excess acid required 36.29 mL of 0.1064 molar sodium hydroxide for complete titration to a phenolphthalein end point. What is the weight percent of calcite in the limestone?a student was titrating a solutin of acitic acid with a sodium hydroxid solution. determin the ph at the equivilance point. do this by constructing a bca table, constructing and ice table, writing the equlibrium constant expresion and find the ph. the ka for ch3cooh is 1.8e-5. a 50ml solutin of 0.3 m of ch3cooh was titrated with 0.3 m of naoh.
- The data below is titration of Na2CO3 with HCl which is a diprotic titration curve. From the endpoint determinations, calculate the concentration of the carbonate solution if the [HCl] was 0.1036 M. use 10.00 mL of carbonate solution for the calculations PH Burett vol added 11.30 0.00 0.00 10.80 0.98 0.98 10.51 1.95 0.97 10.28 3.00 2.02 10.10 3.99 3.01 9.92 4.99 4.01 9.74 5.95 4.97 9.54 6.97 5.99 9.28 7.95 6.97 8.86 8.97 7.99 8.70 9.18 8.2 8.52 9.35 8.37 8.39 9.49 8.51 8.26 9.60 8.62 8.09 9.71 8.73 7.97 9.82 8.84 7.90 9.90 8.92 7.93 10.21 9.23 7.87 10.32 9.34 7.84 10.42 9.44 7.75 10.53 9.55 7.65 10.63 9.65 7.57 10.75 9.77 7.53 10.85 9.87 7.49 10.96 9.98 7.05 11.95 10.97 6.76 12.99 12.01 6.56 13.98 13 6.39 14.93 13.95 6.24 15.98 15 6.07 16.98 16 6.21 17.05 16.07 6.24 17.15 16.17 6.25 17.25 16.27 6.30 17.32 16.34 6.37 17.40 16.42 6.41 17.50 16.52 6.46 17.58 16.6 6.47 17.63 16.65 6.45 17.72 16.74 6.44…Molarity of titrant (NaOH): 0.4550 M HC2H3O2 (aq) + NaOH (aq) → NaC2H3O2 (aq) + H2O (l) Trial # First Second Third Fourth Initial buret reading 0.15 mL 2.43 mL 1.32 mL 0.58 mL Final buret reading 18.62 mL 20.87 mL 20.03 mL 19.14 mL Volume of titrant used 18.47 mL 18.44 mL 18.71 mL 18.56 mL 4) Calculate the molarity of the acetic acid in the vinegar solution (Show your work). use FW for moles-->grams acetic acid. Molarity acetic acid = _____________ M 5) Calculate the weight % of acetic acid in the vinegar. How does this compare with the % listed on the label (5.00%)? (For this calculation assume that density of vinegar is 1.03 g/mL and of course, show your work). Weight % = ___________ 6) If you didn’t get the same weight % of acetic acid as listed on the vinegar label (5.00 %), what are two things (be specific) that could’ve happened during the experiment that could explain the variation from the expected weight %? To do…Titration of 10.00 mL of a 0.444M solution of HCI with a solution of LiSH required 23.2 mL of the LiSH solution to reach the end point. What is the molar concentration of LiSH? HCL + LiSH -> LiCI+H2S a. 0.191M b. 0.223 C. 0.546 d. 0.229
- Titration of (4.890x10^-1) grams of monoprotic weak acid (dissolved in KCl) required (2.44000x10^1) mL of (2.12000x10^-1) molar NaOH solution to reach equivalence. Based on this information, how many moles of weak acid are present in this solution? Format your answer with correct number of sig figs. Use scientific notation.An automatic titration of Cola product was analyzed and the following results were produced: Volume of Standardized NaOH Titrant used to achieve the first equivalence point in titration: Trial #1: 1.300 mL Trial #2: 1.137 mL Trial #3: 1.140 mL May you explain why trial #1 was skewed; for example, if pipetting of the 25.00 mL was done incorrectly, how would the molarity change if the volume examined was supposed to be 24.50 mL?The potentiometric titration data of 2,422 mmol chloride ion with 0.1000 M AgNO3 are as follows. What should the x and y axis values be to find the turning point using the first derivative?

