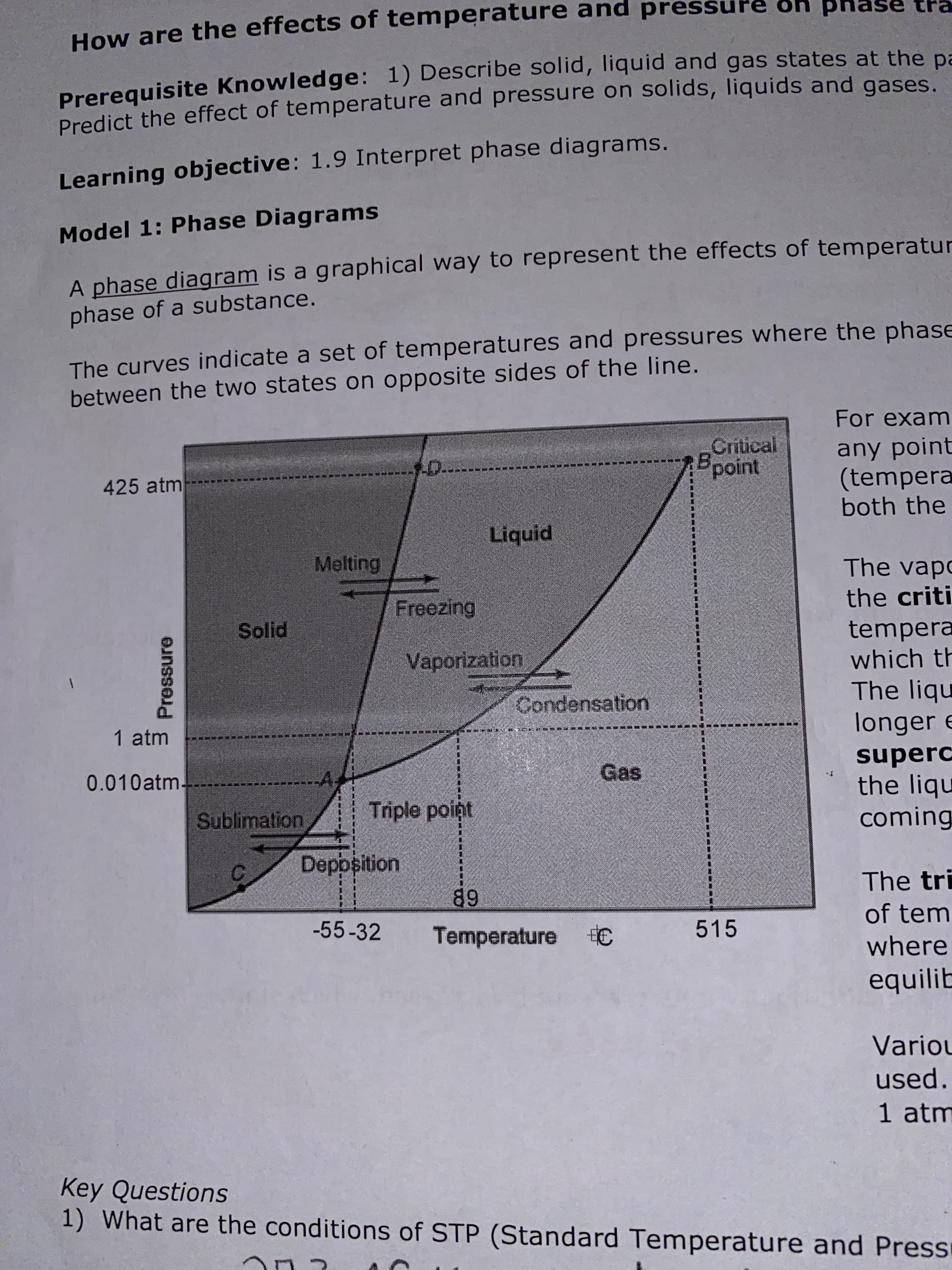
phase How are the effects of temperature and press Prerequisite Knowledge: 1) Describe solid, liquid and gas states at the pa Predict the effect of temperature and pressure on solids, liquids and gases. Learning objective: 1.9 Interpret phase diagrams. Model 1: Phase Diagrams A phase diagram is a graphical way to represent the effects of temperatur phase of a substance. The curves indicate a set of temperatures and pressures where the phase between the two states on opposite sides of the line. For exam Critical Bpoint any point (tempera both the D.. 425 atm Liquid Melting The vapo the criti Freezing tempera which th The liqu longer e Solid Vaporization Condensation 1 atm superc the liqu coming Gas A, 0.010atm. Triple point Sublimation Deposition The tri 89 of tem -55-32 515 Temperature C where equilib Variou used. 1 atm Key Questions 1) What are the conditions of STP (Standard Temperature and Press Pressure A Describe the equilibrium that exists along curve AB at the particulate level. What does it mean to be "at equilibrium" in this context? The particles 5) What are the conditions of temperature and pressure for the triple point? The triple point and pressure where all three phases 0quilibrium. 6) What are the conditions for the critical point? The critical point is is the condition of Hemperarure of temperature exist at the terriperature the Mich tue Cemnot and pressure above be liqu'fied. Tus occurs at 425 atmo and S15°C. 7) At a constant pressure of 0.001 atm, what phase transition(s) occur(s) as the temperature is increased from -273 to 500 °C? 8) At a constant pressure of 5 atm, what phase transition(s) occur as the temperature is increased from -273 to 500 °C.? 9) At a constant temperature of 70°C, what phase transition(s) occur(s) as the pressure in increased? 10) Examine the fusion (freezing-melting) curve. As pressure is increased, which state is favored, liquid or solid? The liquid state is favored. 2.
phase How are the effects of temperature and press Prerequisite Knowledge: 1) Describe solid, liquid and gas states at the pa Predict the effect of temperature and pressure on solids, liquids and gases. Learning objective: 1.9 Interpret phase diagrams. Model 1: Phase Diagrams A phase diagram is a graphical way to represent the effects of temperatur phase of a substance. The curves indicate a set of temperatures and pressures where the phase between the two states on opposite sides of the line. For exam Critical Bpoint any point (tempera both the D.. 425 atm Liquid Melting The vapo the criti Freezing tempera which th The liqu longer e Solid Vaporization Condensation 1 atm superc the liqu coming Gas A, 0.010atm. Triple point Sublimation Deposition The tri 89 of tem -55-32 515 Temperature C where equilib Variou used. 1 atm Key Questions 1) What are the conditions of STP (Standard Temperature and Press Pressure A Describe the equilibrium that exists along curve AB at the particulate level. What does it mean to be "at equilibrium" in this context? The particles 5) What are the conditions of temperature and pressure for the triple point? The triple point and pressure where all three phases 0quilibrium. 6) What are the conditions for the critical point? The critical point is is the condition of Hemperarure of temperature exist at the terriperature the Mich tue Cemnot and pressure above be liqu'fied. Tus occurs at 425 atmo and S15°C. 7) At a constant pressure of 0.001 atm, what phase transition(s) occur(s) as the temperature is increased from -273 to 500 °C? 8) At a constant pressure of 5 atm, what phase transition(s) occur as the temperature is increased from -273 to 500 °C.? 9) At a constant temperature of 70°C, what phase transition(s) occur(s) as the pressure in increased? 10) Examine the fusion (freezing-melting) curve. As pressure is increased, which state is favored, liquid or solid? The liquid state is favored. 2.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter10: Solids, Liquids, And Phase Transitions
Section: Chapter Questions
Problem 66AP
Related questions
Question
Could you help me with #4? I included a picture of the phase diagram that was given.
Transcribed Image Text:phase
How are the effects of temperature and press
Prerequisite Knowledge: 1) Describe solid, liquid and gas states at the pa
Predict the effect of temperature and pressure on solids, liquids and gases.
Learning objective: 1.9 Interpret phase diagrams.
Model 1: Phase Diagrams
A phase diagram is a graphical way to represent the effects of temperatur
phase of a substance.
The curves indicate a set of temperatures and pressures where the phase
between the two states on opposite sides of the line.
For exam
Critical
Bpoint
any point
(tempera
both the
D..
425 atm
Liquid
Melting
The vapo
the criti
Freezing
tempera
which th
The liqu
longer e
Solid
Vaporization
Condensation
1 atm
superc
the liqu
coming
Gas
A,
0.010atm.
Triple point
Sublimation
Deposition
The tri
89
of tem
-55-32
515
Temperature C
where
equilib
Variou
used.
1 atm
Key Questions
1) What are the conditions of STP (Standard Temperature and Press
Pressure

Transcribed Image Text:A Describe the equilibrium that exists along curve AB at the particulate level. What does it mean
to be "at equilibrium" in this context?
The particles
5) What are the conditions of temperature and pressure for the triple point?
The triple point
and pressure where all three phases
0quilibrium.
6) What are the conditions for the critical point?
The critical point is
is the
condition
of Hemperarure
of temperature
exist at
the terriperature
the
Mich tue
Cemnot
and
pressure above
be liqu'fied. Tus occurs at 425 atmo and S15°C.
7) At a constant pressure of 0.001 atm, what phase transition(s) occur(s) as the temperature is
increased from -273 to 500 °C?
8) At a constant pressure of 5 atm, what phase transition(s) occur as the temperature is increased
from -273 to 500 °C.?
9) At a constant temperature of 70°C, what phase transition(s) occur(s) as the pressure in
increased?
10) Examine the fusion (freezing-melting) curve. As pressure is increased, which state is favored,
liquid or solid?
The liquid state is favored.
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning