Phthalic acid (H,C3H,O4, abbreviated H,Ph) is a diprotic acid. Its ionization in water at 25°C_takes place in two steps: H¿Ph(aq) + H20(e)=H;0* (aq) + HPH¯(aq) Ka1 = 1.26 × 10~³ %3D HPH¯(aq) + H,O(e)2H;0*(aq) + Ph² (aq) K22 3.10 × 10¬6 If 0.0100 mol of phthalic acid is dissolved per liter of water, calculate the equilibrium concentrations of H,Ph, HPH¯, Ph²-, and H;O*.
Phthalic acid (H,C3H,O4, abbreviated H,Ph) is a diprotic acid. Its ionization in water at 25°C_takes place in two steps: H¿Ph(aq) + H20(e)=H;0* (aq) + HPH¯(aq) Ka1 = 1.26 × 10~³ %3D HPH¯(aq) + H,O(e)2H;0*(aq) + Ph² (aq) K22 3.10 × 10¬6 If 0.0100 mol of phthalic acid is dissolved per liter of water, calculate the equilibrium concentrations of H,Ph, HPH¯, Ph²-, and H;O*.
Chapter10: Effect Of Electrolytes On Chemical Equilibria
Section: Chapter Questions
Problem 10.4QAP
Related questions
Question
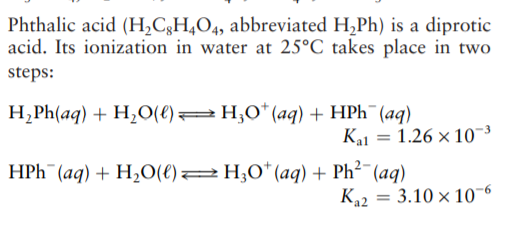
Transcribed Image Text:Phthalic acid (H,C3H,O4, abbreviated H,Ph) is a diprotic
acid. Its ionization in water at 25°C_takes place in two
steps:
H¿Ph(aq) + H20(e)=H;0* (aq) + HPH¯(aq)
Ka1 = 1.26 × 10~³
%3D
HPH¯(aq) + H,O(e)2H;0*(aq) + Ph² (aq)
K22
3.10 × 10¬6

Transcribed Image Text:If 0.0100 mol of phthalic acid is dissolved per liter of
water, calculate the equilibrium concentrations of H,Ph,
HPH¯, Ph²-, and H;O*.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

