PHYSICAL TEST Instructions: Note the SOLUBILITY of each of the samples in the beakers by writing dissolve or did not dissolve. Base on the table for solubility, describe the solubility of SUCROSE in ETHANOL. Answer the highlighted numbers. Reference Sucrose Solution did not dissolved did not dissolved did not dissolved did not dissolved dissolved Slightly soluble Sample A Sample B Beaker 1 (1ml solvent) Beaker 2 (8ml solvent) Beaker 3 (25ml solvent) Beaker 4 (80ml solvent) Beaker 5 (250ml solvent) Solubility Description did not dissolved did not dissolved did not dissolved did not dissolved dissolved did not dissolved did not dissolved did not dissolved dissolved dissolved 1 1. 2. 3. Which of the two samples is comparative to the standard? SOLUBILITY TABLE Descriptive Term Parts of Solvent g/L in water M=400 M=40000 mol/L mol/L in water Required for 1 part of Solute in water Very soluble Freely soluble Soluble s1 21000 22,5 20,025 2,5 to 0,25 1 to 10 10 to 30 0,025 to 0,0025 1000 to 100 100 to 33 0,25 to 0,08 0,0025 to 0,0008 Sparingly soluble Slightly soluble Very slightly soluble Practically insoluble, or Insoluble 30 to 100 33 to 10 0,08 to 0,025 0,0008 to 0,00025 100 to 1000 1000 to 10,000 0,025 to 0,0025 0,0025 to 0,00025 0,000025 to 0,0000025 10 to 1 0,00025 to 0,0000025 1 to 0,1 210,000 S0,1 S0,00025 s0,0000025
PHYSICAL TEST Instructions: Note the SOLUBILITY of each of the samples in the beakers by writing dissolve or did not dissolve. Base on the table for solubility, describe the solubility of SUCROSE in ETHANOL. Answer the highlighted numbers. Reference Sucrose Solution did not dissolved did not dissolved did not dissolved did not dissolved dissolved Slightly soluble Sample A Sample B Beaker 1 (1ml solvent) Beaker 2 (8ml solvent) Beaker 3 (25ml solvent) Beaker 4 (80ml solvent) Beaker 5 (250ml solvent) Solubility Description did not dissolved did not dissolved did not dissolved did not dissolved dissolved did not dissolved did not dissolved did not dissolved dissolved dissolved 1 1. 2. 3. Which of the two samples is comparative to the standard? SOLUBILITY TABLE Descriptive Term Parts of Solvent g/L in water M=400 M=40000 mol/L mol/L in water Required for 1 part of Solute in water Very soluble Freely soluble Soluble s1 21000 22,5 20,025 2,5 to 0,25 1 to 10 10 to 30 0,025 to 0,0025 1000 to 100 100 to 33 0,25 to 0,08 0,0025 to 0,0008 Sparingly soluble Slightly soluble Very slightly soluble Practically insoluble, or Insoluble 30 to 100 33 to 10 0,08 to 0,025 0,0008 to 0,00025 100 to 1000 1000 to 10,000 0,025 to 0,0025 0,0025 to 0,00025 0,000025 to 0,0000025 10 to 1 0,00025 to 0,0000025 1 to 0,1 210,000 S0,1 S0,00025 s0,0000025
Chapter79: Solubility
Section: Chapter Questions
Problem 1P
Related questions
Question
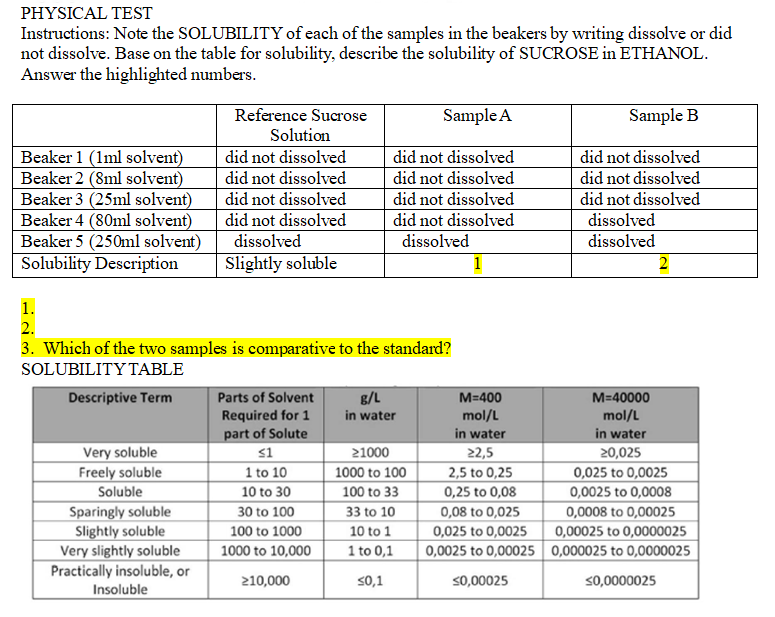
PHYSICAL TEST
Instructions: Note the SOLUBILITY of each of the samples in the beakers by writing dissolve or did not dissolve. Base on the table for solubility, describe the solubility of SUCROSE in ETHANOL. Answer the highlighted numbers.
1.
2.
3. Which of the two samples is comparative to the standard?
SOLUBILITY TABLE (see tables)
Transcribed Image Text:PHYSICAL TEST
Instructions: Note the SOLUBILITY of each of the samples in the beakers by writing dissolve or did
not dissolve. Base on the table for solubility, describe the solubility of SUCROSE in ETHANOL.
Answer the highlighted numbers.
Reference Sucrose
Sample A
Sample B
Solution
did not dissolved
Beaker 1 (1ml solvent)
Beaker 2 (8ml solvent)
Beaker 3 (25ml solvent)
Beaker 4 (80ml solvent)
Beaker 5 (250ml solvent)
Solubility Description
did not dissolved
did not dissolved
did not dissolved
did not dissolved
did not dissolved
did not dissolved
did not dissolved
dissolved
did not dissolved
did not dissolved
dissolved
did not dissolved
dissolved
dissolved
Slightly soluble
1
2
1.
2.
3. Which of the two samples is comparative to the standard?
SOLUBILITY TABLE
g/L
M=40000
mol/L
in water
20,025
0,025 to 0,0025
0,0025 to 0,0008
0,0008 to 0,00025
0,00025 to 0,0000025
Descriptive Term
Parts of Solvent
M=400
Required for 1
part of Solute
in water
mol/L
in water
Very soluble
Freely soluble
Soluble
s1
1 to 10
21000
22,5
1000 to 100
2,5 to 0,25
10 to 30
100 to 33
0,25 to 0,08
Sparingly soluble
Slightly soluble
Very slightly soluble
Practically insoluble, or
30 to 100
33 to 10
0,08 to 0,025
100 to 1000
10 to 1
0,025 to 0,0025
1000 to 10,000
1 to 0,1
0,0025 to 0,00025 0,000025 to 0,0000025
210,000
s0,1
S0,00025
s0,0000025
Insoluble
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning